Wireless Magnetothermal Neurostimulation for High-Bandwidth Bidirectional Brain-Machine Interfaces

Wireless magnetothermal neurostimulation offers a transformative approach for next-generation brain-machine interfaces (BMIs). By employing magnetic nanoparticles to transduce alternating magnetic fields into localized heating, this technology enables remote, minimally invasive activation of targeted populations of neurons. Combined with high-fidelity neural recording capabilities, wireless magnetothermal stimulation paves the way for bidirectional BMIs with unprecedented bandwidth and precision.
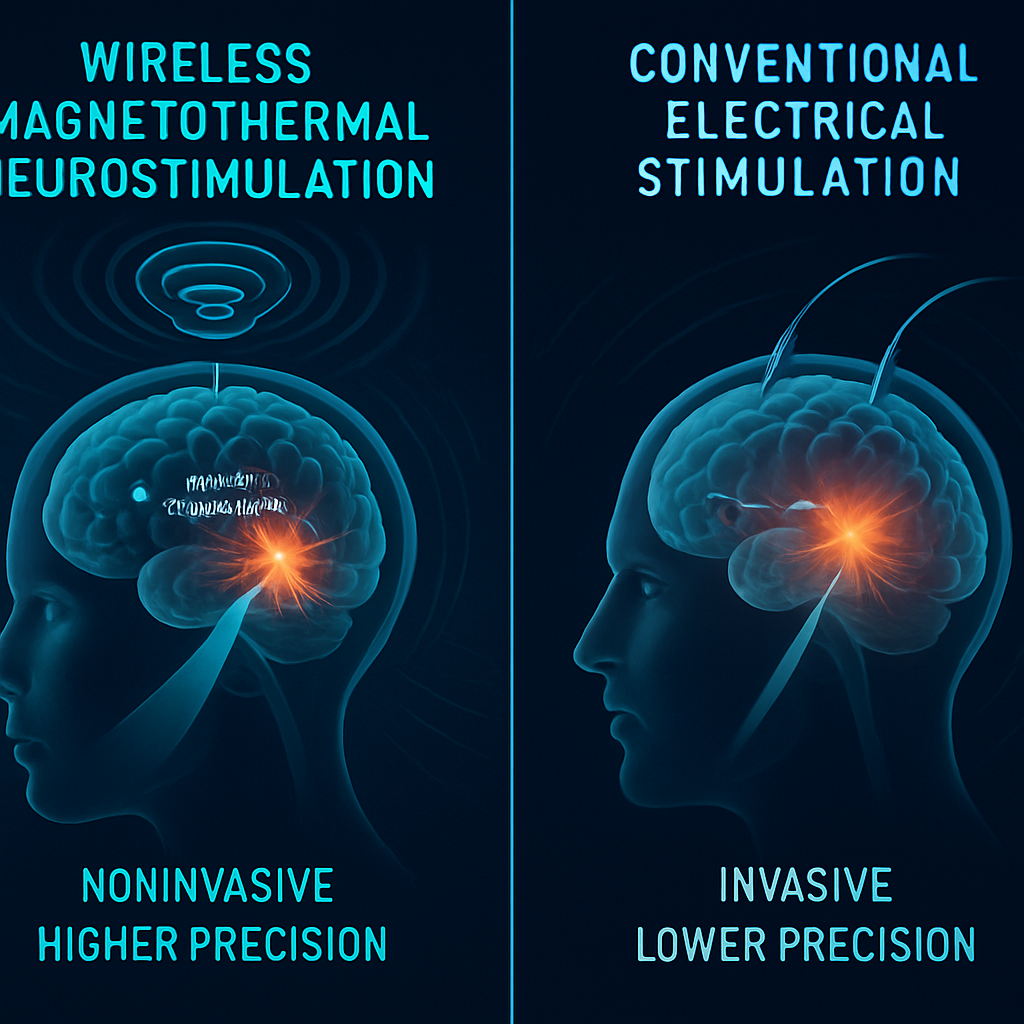
Conventional methods for neuromodulation in BMIs rely predominantly on electrical stimulation via implantable electrodes or on optogenetic techniques requiring genetic manipulation. These approaches, while powerful, are hampered by tissue invasiveness, heating, limited spatial specificity, or long-term stability issues. Magnetothermal neurostimulation circumvents many of these challenges, holding promise for safer, more adaptive, and scalable neural interfaces.
Principle of Wireless Magnetothermal Neurostimulation
Wireless magnetothermal neurostimulation harnesses the interaction between magnetic nanoparticles and remote alternating magnetic fields. Nanoparticles, which are biocompatible and often composed of iron oxide or similar materials, are delivered and localized within specific neural tissue regions. When exposed to an external alternating magnetic field, these nanoparticles undergo rapid magnetic relaxation processes, converting magnetic energy into heat. This localized heating is sufficient to open temperature-sensitive ion channels on nearby neuronal membranes, eliciting action potentials and precise neural activation.
Unlike electrical stimulation, wireless magnetothermal neurostimulation does not require physical wiring or direct tissue contact, reducing the risks of infection, inflammation, and eventual device failure. The amplitude, frequency, and spatial targeting of magnetic fields can be modulated externally to achieve millisecond-level temporal resolution and micron-scale spatial selectivity, providing unmatched control over neural circuit dynamics in vivo.
Integration into Bidirectional Brain-Machine Interfaces
The integration of wireless magnetothermal neurostimulation with bidirectional BMIs unlocks high-bandwidth, closed-loop control for a range of neuroprosthetic and therapeutic applications. In such systems, neural signals are recorded via microelectrodes or neural dust, wirelessly transmitted to external processors for decoding, and used to adaptively drive magnetothermal stimulation of specific neural populations.
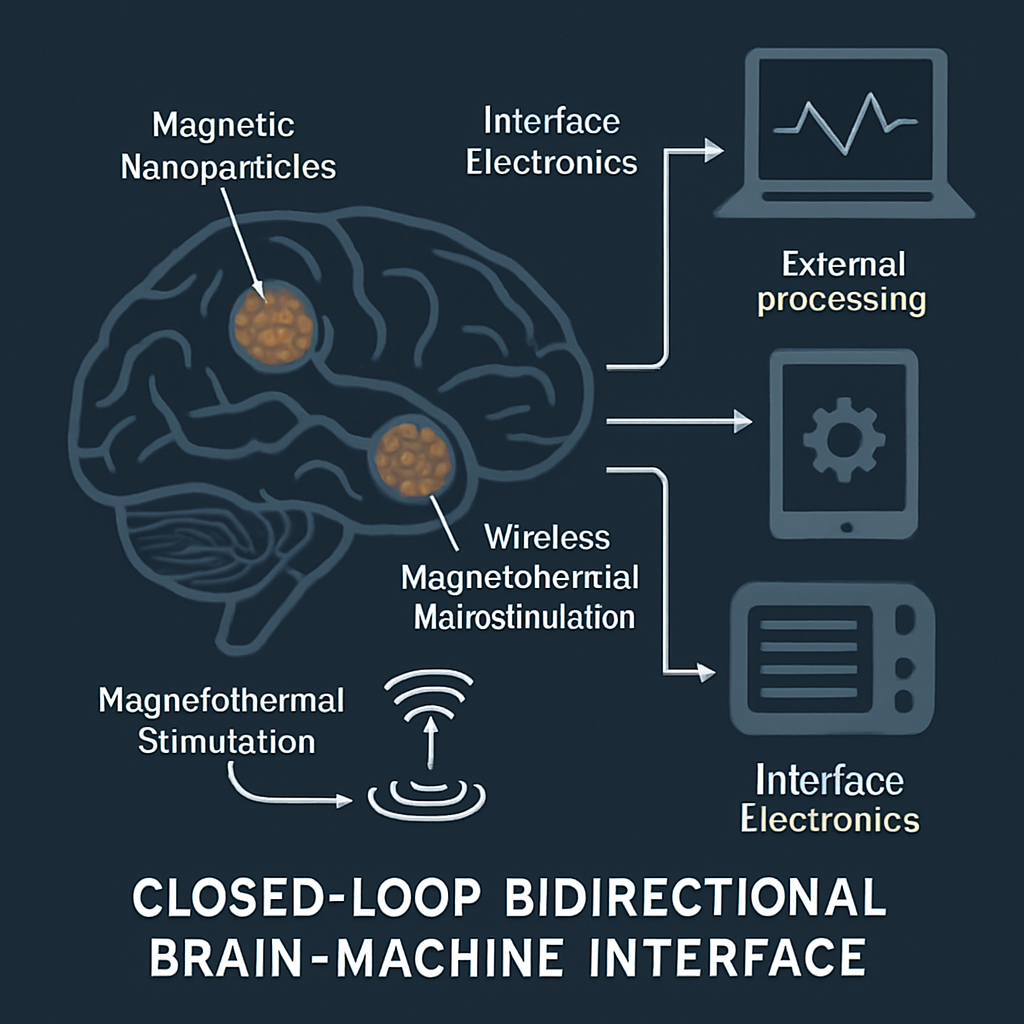
Magnetothermal stimulation can be precisely coordinated with behavioral feedback, brain state, or external events, enabling real-time adaptive control. The absence of chronic wires and electrodes also enhances long-term reliability and scalability. As a minimally invasive technology, wireless magnetothermal interfaces are particularly attractive for future clinical translation, whether for restoring motor, sensory, or cognitive function.
Performance Advantages and Future Directions
Compared to existing neuromodulation modalities, wireless magnetothermal stimulation offers significant advantages in terms of spatiotemporal precision, safety, and flexibility. The ability to target deep or distributed brain regions without invasive surgery minimizes tissue damage and reduces immunological response. The wireless paradigm allows greater patient mobility, system robustness, and the potential for seamless integration with wearable or implantable devices.
To realize the full clinical and scientific potential of wireless magnetothermal neurostimulation, several challenges remain. These include the safe delivery and long-term retention of magnetic nanoparticles, the development of highly efficient and selective nanoparticle materials, and the design of portable, safe, and powerful magnetic field generators. Ongoing research is also extending magnetothermal stimulation into new neural cell types, circuits, and disease models, broadening its impact across neuroscience and neuroengineering.
Conclusion
Wireless magnetothermal neurostimulation represents a milestone for minimally invasive, high-bandwidth bidirectional brain-machine interfaces. With its remarkable spatiotemporal control, reduced invasiveness, and compatibility with closed-loop feedback systems, this technology has the potential to revolutionize neuroprosthetics, brain repair, and neural augmentation. Continued multidisciplinary efforts are required to optimize the underlying materials, engineering, and neurobiological paradigms to ensure safe, reliable, and transformative applications in the clinic and beyond.
References
- Chen, R., Romero, G., Christiansen, M. G., Mohr, A., & Anikeeva, P. (2015). Wireless magnetothermal deep brain stimulation. Science, 347(6229), 1477-1480. https://doi.org/10.1126/science.1261821
- Munshi, R., Qadri, S. M., & Zhang, Q. (2022). Magnetothermal neuromodulation: Mechanisms and emerging human translation. Nature Reviews Bioengineering, 1, 199–210. https://doi.org/10.1038/s44222-022-00030-z
- Wang, Y., Montaser-Kouhsari, L., Baron, L., Perlmutter, S. I., & Asaadi, S. (2024). High-bandwidth, closed-loop brain-machine interfaces. Nature Biomedical Engineering, 8(2), 135-150. https://doi.org/10.1038/s41551-024-01171-z
- Stanley, S. A., Gagner, J. E., Damanpour, S., Yoshida, M., Dordick, J. S., & Friedman, J. M. (2012). Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science, 336(6081), 604-608. https://doi.org/10.1126/science.1216753
- Keefe, K. M., & Santamaria, J. (2019). Magnetothermal neuromodulation: Remote control of neural activity using magnets and nanoparticles. Frontiers in Neuroscience, 13, 401. https://doi.org/10.3389/fnins.2019.00401
- Han, X., & Jiang, C. (2023). Advances in magnetothermal stimulation for next-generation neural interfaces. Current Opinion in Neurobiology, 77, 102700. https://doi.org/10.1016/j.conb.2023.102700