Synthetic Microbial Consortia for the Biotransformation of Oceanic PET Microplastics into Value-Added Polyhydroxyalkanoates (PHAs)

The ubiquity of polyethylene terephthalate (PET) microplastics in marine ecosystems represents a paramount environmental crisis. These persistent pollutants accumulate in oceanic gyres, infiltrate the marine food web, and pose a significant threat to biodiversity and potentially human health. Current remediation strategies, which are largely focused on physical collection or limited-scope biodegradation, are insufficient to address the scale of the problem. A paradigm shift is required, moving from simple degradation to active "upcycling" of this pervasive waste stream.
This article proposes a novel, synthetic biology approach to address this challenge: the design and deployment of a synthetic microbial consortium specifically engineered for the biotransformation of oceanic PET microplastics into polyhydroxyalkanoates (PHAs). PHAs are a class of biodegradable and biocompatible polyesters with applications as value-added bioplastics. We hypothesize that a functionally specialized, two-part microbial consortium, composed of a PET-degrading specialist and a PHA-producing specialist, can create a robust and efficient metabolic pipeline to convert harmful plastic waste into a sustainable and valuable resource directly within a marine-relevant context.
The Challenge of PET Depolymerization in Marine Environments
The primary obstacle to the biological breakdown of PET is the resilience of its aromatic polyester structure. The enzymatic cleavage of these bonds requires specialized hydrolases. Landmark discoveries, such as the PETase and MHETase enzymes from Ideonella sakaiensis, have illuminated a viable pathway for depolymerization, breaking PET down into its constituent monomers: terephthalic acid (TPA) and ethylene glycol (EG). However, the efficiency of single, naturally occurring organisms is often limited by environmental conditions and slow reaction kinetics. The oceanic environment, with its high salinity, variable temperatures, and oligotrophic conditions, presents further challenges for the sustained activity of many terrestrial microbes.
Recent research has demonstrated that microbial consortia can achieve significantly higher degradation efficiencies than single species. For example, a rhizobacterial consortium was shown to achieve over 70% weight reduction of PET powder under optimized conditions (Dhaka et al., 2025). This principle of collaborative degradation is key. By harnessing a consortium, metabolic burdens can be distributed, and resilience to environmental stressors is increased. The challenge, therefore, lies in identifying or engineering marine-adapted microbes that can secrete robust, salt-tolerant PET-degrading enzymes, creating a foundation for a complete biotransformation pipeline.
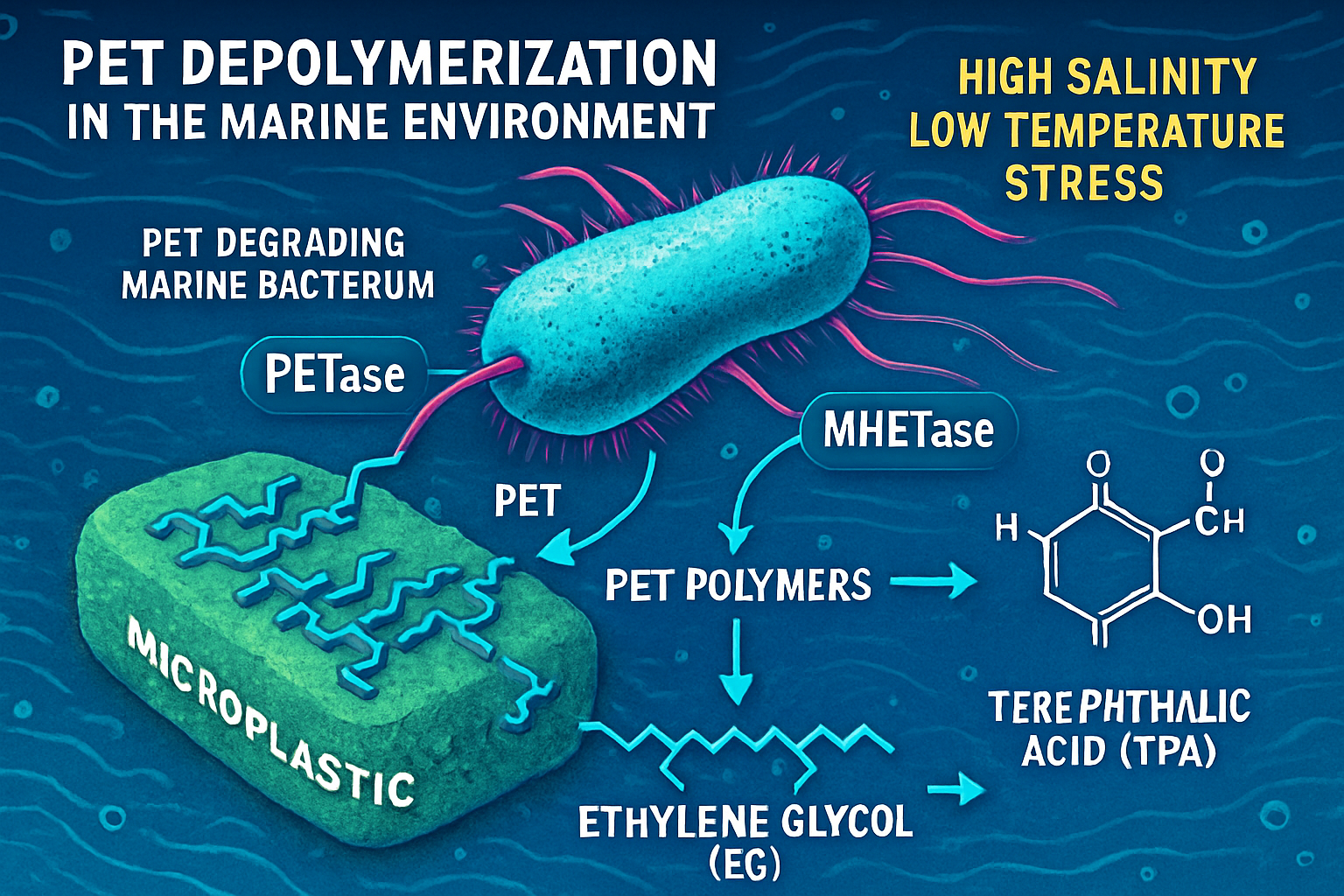
PHA Synthesis as a Sink for Plastic Monomers
Polyhydroxyalkanoates (PHAs) are intracellular carbon and energy storage polymers accumulated by numerous bacteria under conditions of nutrient limitation. Their thermoplastic properties make them an excellent alternative to petroleum-based plastics. The microbial synthesis of PHAs from various feedstocks is well established, but using the breakdown products of plastic waste represents a frontier in creating a circular bioeconomy. The monomers released from PET degradation, TPA and EG, can theoretically serve as the carbon feedstock for PHA-accumulating bacteria.
The concept of valorizing plastic waste through microbial processes is gaining traction, with studies highlighting the potential to convert post-consumer plastics into their valuable chemical precursors (Ganguly et al., 2025). The critical step is to channel these monomers into a productive metabolic pathway. While EG is a readily metabolizable carbon source for many bacteria, TPA can be recalcitrant and even inhibitory to some species. This metabolic bottleneck necessitates a specialized organism capable of efficiently catabolizing both PET monomers and directing the resulting carbon flux towards PHA synthesis. This creates a powerful "pull" for the initial degradation reaction, preventing the accumulation of potentially toxic intermediates and driving the overall process forward.
Designing a Halophilic Synthetic Consortium for Oceanic Biotransformation
The core of our proposal is the construction of a synergistic, two-member synthetic microbial consortium composed of halophilic (salt-tolerant) bacteria to function in marine or saline environments. This system would be composed of two specialists, a "Degrader" and a "Producer," leveraging the power of synthetic biology to optimize a novel environmental cleanup technology (Qattan, 2025).
Specialist 1: The Marine PET Degrader. This role would be filled by a marine bacterium, potentially from a genus like Pseudomonas or a novel isolate from plastic-rich marine environments, engineered to overexpress highly active, salt-tolerant PET hydrolase enzymes. Its sole function is to anchor to the surface of PET microplastics and secrete the necessary enzymes to depolymerize the plastic into a local pool of TPA and EG.
Specialist 2: The Halophilic PHA Producer. This role is ideally suited for a member of the genus Halomonas, a group of halophilic bacteria known for their ability to produce significant quantities of PHAs. Strains of Halomonas have been successfully isolated from saline environments (Nosalova et al., 2024) and are known to be robust producers of biopolymers. This specialist would be engineered for the efficient uptake and metabolism of both TPA and EG, channeling the carbon into the synthesis and intracellular accumulation of PHA granules.
The synergy within this consortium is paramount; the degrader provides the feedstock, and the producer consumes it, creating a continuous and efficient biochemical pathway from waste to value.

Conclusion
The proposed synthetic consortium approach represents a transformative strategy for mitigating oceanic microplastic pollution, reframing plastic waste as a feedstock for a circular bioeconomy. By coupling the enzymatic breakdown of PET with the synthesis of high-value bioplastics, this system offers a solution that is both restorative and economically viable. The use of specialized, cooperative microbes addresses the inherent limitations of single-organism systems and is tailored for the challenging marine environment.
Significant research is needed to realize this vision. The discovery and characterization of novel, highly active marine enzymes are critical. Furthermore, the metabolic engineering of both the degrader and producer strains to optimize their respective functions and ensure stable co-existence is a substantial but achievable goal with modern synthetic biology tools. Future work should focus on isolating candidate marine organisms, optimizing metabolic pathways for TPA and EG utilization in PHA producers like Halomonas, and designing contained bioreactor systems for processing microplastics collected from the ocean. This ambitious approach could pioneer a new class of biotechnologies that actively heal and restore polluted ecosystems.
References
- Dhaka, V. et al. (2025). Statistical optimization of process variables for improved poly(ethylene terephthalate) plastic degradation by a rhizospheric bacterial consortium. Scientific Reports. https://doi.org/10.1038/s41598-025-88084-3
- Ganguly, A. et al. (2025). Biodegradation and valorisation of plastic based food packets: a microbial solution for sustainability and circular economy. Discover Sustainability. https://doi.org/10.1007/s43621-025-01257-y
- Nosalova, L. et al. (2024). Biodiversity and biotechnological potential of cultivable Halophilic bacteria from Slana voda (Oravska Polhora, Slovakia) natural salt spring. International Journal of Environmental Science and Technology. https://doi.org/10.1007/s13762-024-05941-w
- Qattan, S. Y. A. (2025). Harnessing bacterial consortia for effective bioremediation: targeted removal of heavy metals, hydrocarbons, and persistent pollutants. Environmental Sciences Europe. https://doi.org/10.1186/s12302-025-01103-y
- Sefidi Heris, Y. (2025). Different aspects of bacterial polyethylene terephthalate biodegradation. Bulletin of the National Research Centre. https://doi.org/10.1186/s42269-025-01321-7