Synthetic Glycobiology: Engineering Custom Glycans for Therapeutic Applications
Glycosylation, the enzymatic attachment of sugar moieties (glycans) to proteins and lipids, is a fundamental post-translational modification that profoundly influences a wide array of biological processes, including protein folding, cell-cell recognition, immune responses, and pathogen-host interactions. The immense structural diversity of glycans translates into a complex 'glycocode' that modulates the function and fate of glycoconjugates. Synthetic glycobiology has emerged as a powerful interdisciplinary field that combines principles of chemistry, biology, and engineering to precisely construct and manipulate these complex carbohydrate structures. This capability to design and synthesize custom glycans and glycoconjugates opens unprecedented avenues for developing novel therapeutic interventions for a multitude of diseases, ranging from cancer and infectious diseases to metabolic and autoimmune disorders.
The therapeutic potential of engineered glycans stems from their ability to modulate biological pathways with high specificity. By tailoring glycan structures, researchers can enhance the efficacy of protein-based drugs, design novel vaccines that elicit robust and targeted immune responses, develop sensitive diagnostic tools, and create innovative biomaterials. This article reviews recent advancements in synthetic glycobiology, focusing on strategies for engineering custom glycans and their diverse applications in the therapeutic landscape. We explore cutting-edge tools and techniques enabling precise glycan synthesis and delve into the challenges and future directions of this rapidly evolving field.
Chemoenzymatic Synthesis: A Hybrid Approach to Complex Glycans
The synthesis of complex glycans presents a significant chemical challenge due to the stereochemical complexity and the need for regioselective glycosidic bond formation. Purely chemical synthesis, while powerful, can be laborious and often requires extensive protecting group manipulations. Chemoenzymatic synthesis has emerged as a highly efficient and versatile alternative, leveraging the specificity of enzymes for glycosidic bond formation in concert with the flexibility of chemical synthesis for constructing building blocks and scaffolds. This hybrid approach allows for the construction of intricate glycan structures, including those found on glycoproteins and glycolipids, with remarkable precision and efficiency.
Recent advances in enzyme discovery and engineering have significantly expanded the chemoenzymatic toolbox. Glycosyltransferases, the enzymes responsible for catalyzing the formation of glycosidic linkages in nature, are central to this approach. Researchers have developed methods for producing these enzymes in recombinant form and have engineered their substrate specificity and catalytic activity. For example, endo-β-N-acetylglucosaminidases (ENGases) have been engineered for efficient transglycosylation, enabling the remodeling of N-glycans on glycoproteins (Shinoda, C. et al., 2025). This allows for the attachment of custom-designed glycans to therapeutic proteins, potentially improving their pharmacokinetic properties, stability, and efficacy. Furthermore, innovative strategies using diazo-thioglycoside donors activated by earth-abundant iron or photosensitizer-free blue light conditions are providing orthogonal and stereoselective glycosylation methods, expanding the repertoire for complex glycan assembly (Singh, S. P. et al., 2025).
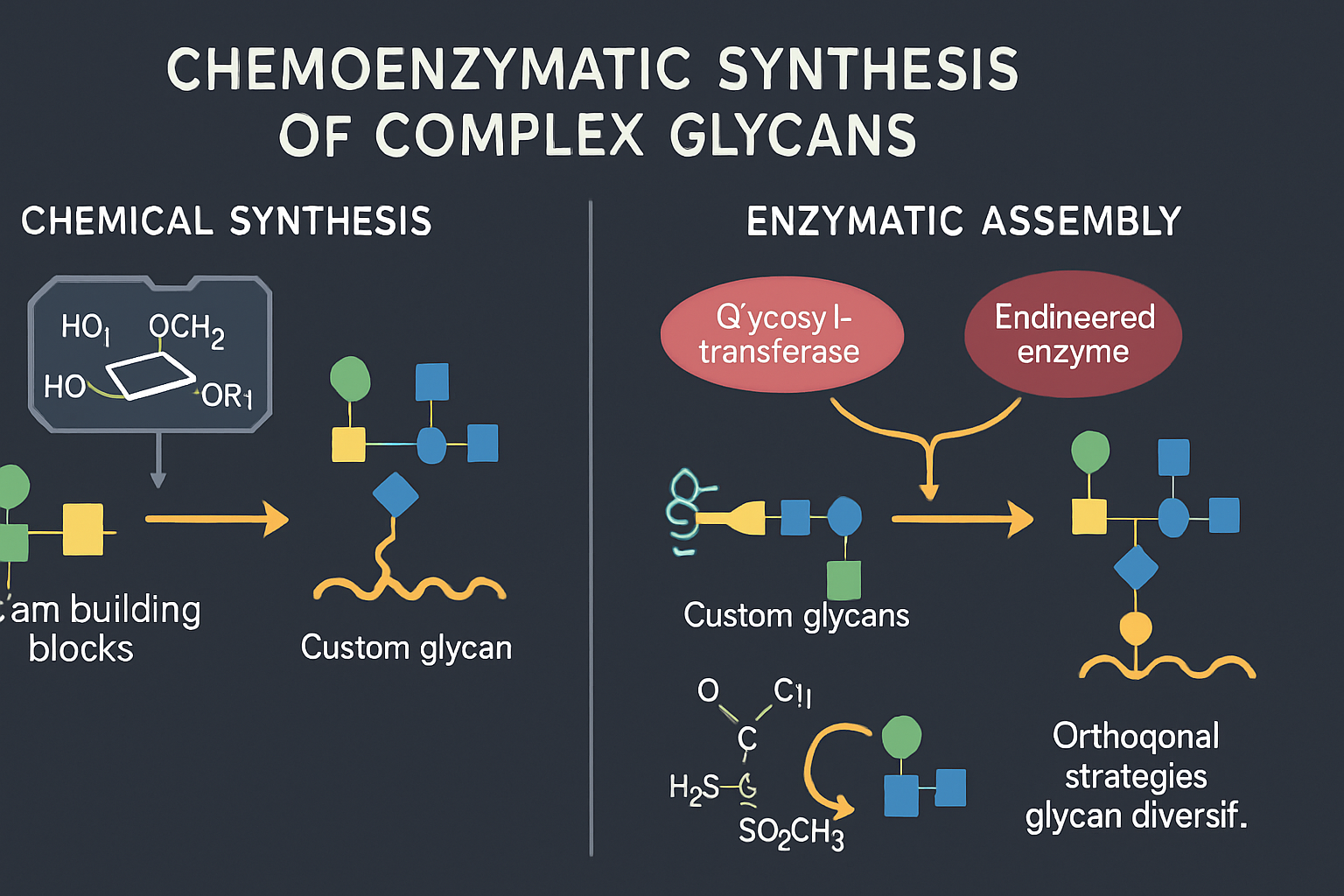
The power of chemoenzymatic synthesis is evident in the development of tailored glycans for vaccine development. For instance, the conserved Group A Carbohydrate of Streptococcus pyogenes, a rhamnose polysaccharide (RhaPS), has been recombinantly produced as a glycoconjugate vaccine candidate within Escherichia coli cells, demonstrating the potential for large-scale, cost-effective production of complex glycan-based vaccines (Ajay Castro, S. et al., 2025). Similarly, the ability to modify the glycosylation profile of viral antigens, such as the SARS-CoV-2 spike protein, has been shown to significantly impact the elicited immune response, highlighting the importance of precise glycan engineering in vaccine design (Renner, T. M. et al., 2025). The continued development of novel enzymes and synthetic methodologies will further empower chemoenzymatic approaches for creating increasingly complex and functionally optimized glycans for therapeutic use.
Glycoengineering Therapeutic Proteins: Enhancing Efficacy and Safety
Glycosylation is a critical quality attribute of many protein-based therapeutics, particularly monoclonal antibodies (mAbs) and other recombinant glycoproteins. The type and distribution of glycans on these proteins can profoundly affect their stability, solubility, serum half-life, immunogenicity, and effector functions. Glycoengineering, the targeted modification of glycosylation pathways in host cells or in vitro, offers a means to optimize these properties, leading to improved therapeutic outcomes.
One key area of focus is the manipulation of N-glycosylation in mammalian cell lines, such as Chinese Hamster Ovary (CHO) cells, which are widely used for producing therapeutic proteins. Strategies to modulate the glycosylation machinery within these cells, for example, by overexpressing or knocking down specific glycosyltransferases or by using glycosylation inhibitors, can lead to the production of glycoproteins with more homogeneous and desirable glycan profiles. For instance, optimizing culture media and supplements can influence glycosylation patterns, as demonstrated in the production of the VRC01 mAb (Lee, J. et al., 2025). Furthermore, engineering the unfolded protein response (UPR) in CHO cells has been shown to improve the production and glycosylation of IgGs by mitigating ER stress (Rives, D. et al., 2024).
Beyond cell-based approaches, in vitro glycoengineering techniques, such as enzyme-mediated glycan remodeling, are gaining prominence. Immobilized enzyme cascades are being developed for sequential glycosylation reactions to create bespoke, controlled N-linked glycan structures on proteins derived from various cellular systems (Makrydaki, E. et al., 2024). This allows for 'humanization' of glycan profiles, reducing potential immunogenicity and enhancing therapeutic function. For example, the galactosylation profile of IgGs can be significantly enhanced using immobilized β-1,4-galactosyltransferase, leading to near-homogeneous terminal galactosylation. The ability to tailor glycan structures, such as sialylation and fucosylation, is particularly important for antibody-drug conjugates (ADCs) and immunotherapies, as these modifications can influence antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) (Wang, R. et al., 2025; Tian, M. et al., 2025).

Glycan-Based Diagnostics and Biomarkers
Alterations in glycosylation are hallmarks of many diseases, including cancer, inflammatory disorders, and infectious diseases. These aberrant glycan structures, often found on cell surfaces or secreted glycoproteins, can serve as valuable biomarkers for early detection, diagnosis, prognosis, and monitoring treatment responses. Synthetic glycobiology plays a crucial role in developing tools and methods to identify and characterize these disease-associated glycans and to translate these findings into clinically useful diagnostic assays.
The profiling of IgG N-glycans has emerged as a particularly promising area for biomarker discovery. Changes in IgG galactosylation, sialylation, and fucosylation have been linked to various conditions, including insulin resistance (Chen, X. et al., 2025), gastric cancer (Xu, T. et al., 2025), axial spondyloarthritis (Xu, X. et al., 2025), and even aging (Vinicki, M. et al., 2024). High-throughput analytical techniques, such as mass spectrometry and liquid chromatography, coupled with sophisticated data analysis, are enabling the identification of specific glycan signatures associated with disease states (Liu, S. et al., 2024; Jager, S. et al., 2025). For example, specific serum N-glycan signatures have been identified that can differentiate between major gastrointestinal cancers (Liu, S. et al., 2024), and urinary extracellular vesicle N-glycomics shows promise for non-invasive bladder cancer screening (Li, Y. et al., 2025).
Synthetic glycans are also vital for developing diagnostic tools. Defined glycan structures can be used to create glycan microarrays for probing antibody responses or identifying lectin binding patterns associated with disease. For example, lectin microarrays have been used to identify unique glycomic features in parathyroid carcinoma tissues (Zheng, Q. et al., 2024). Furthermore, methods for purifying and analyzing glycans, such as the use of cellulose-functionalized magnetic beads (Wan, C. et al., 2024) and cleavable tags for large-scale N-glycan production (Zhang, Q. et al., 2025), are accelerating the pace of glycan biomarker discovery and validation. The integration of glycomic data with other omics data and clinical information is expected to yield more robust and clinically actionable diagnostic and prognostic models (Lin, P. et al., 2025; Wang, M. et al., 2024). The GlycoDash web application represents an effort to streamline the curation and analysis of large glycoproteomics datasets, facilitating such integrative studies (Pongracz, T. et al., 2025).
Conclusion
Synthetic glycobiology has matured into a vibrant and impactful field, providing essential tools and strategies for dissecting the complex world of glycans and harnessing their therapeutic potential. The ability to engineer custom glycans with precision has revolutionized the development of biopharmaceuticals, vaccines, and diagnostics. Chemoenzymatic synthesis, host cell glycoengineering, and in vitro glycan remodeling techniques are continually expanding the accessibility and diversity of tailored glycan structures. These advancements are leading to therapeutic proteins with enhanced efficacy and safety profiles, novel vaccine candidates that elicit targeted immune responses, and highly sensitive glycan-based biomarkers for a myriad of diseases.
Despite significant progress, challenges remain. The large-scale, cost-effective synthesis of complex glycans and the full elucidation of structure-function relationships for many glycan epitopes are ongoing endeavors. Future research will likely focus on deeper integration of computational tools, automation, and high-throughput screening to accelerate discovery and development. The exploration of novel biological contexts for glycan engineering, such as microbiome modulation and the development of glycan-based immunotherapies targeting the tumor microenvironment (Imani, S. et al., 2025), will open new therapeutic frontiers. As our understanding of the intricate roles of glycans in biological systems continues to grow, synthetic glycobiology will undoubtedly play an increasingly central role in shaping the future of medicine.
References
- Ajay Castro, S. et al. (2025). Recombinant production platform for Group A Streptococcus glycoconjugate vaccines. npj Vaccines.
- Bhatta, R. et al. (2025). Injectable extracellular vesicle hydrogels with tunable viscoelasticity for depot vaccine. Nature Communications.
- Chen, X. et al. (2025). IgG N-glycosylation contributes to different severities of insulin resistance: implications for 3P medical approaches. EPMA Journal.
- Dewaker, V. et al. (2025). Revolutionizing oncology: the role of Artificial Intelligence (AI) as an antibody design, and optimization tools. Biomarker Research.
- Gong, Z. et al. (2025). Harnessing engineered extracellular vesicles for enhanced therapeutic efficacy: advancements in cancer immunotherapy. Journal of Experimental & Clinical Cancer Research.
- Imani, S. et al. (2025). Reprogramming the breast tumor immune microenvironment: cold-to-hot transition for enhanced immunotherapy. Journal of Experimental & Clinical Cancer Research.
- Jager, S. et al. (2025). In-depth plasma N-glycoproteome profiling using narrow-window data-independent acquisition on the Orbitrap Astral mass spectrometer. Nature Communications.
- Jaroentomeechai, T. et al. (2018). Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nature Communications.
- Kay, E. J. et al. (2022). Engineering a suite of E. coli strains for enhanced expression of bacterial polysaccharides and glycoconjugate vaccines. Microbial Cell Factories.
- Lee, J. et al. (2025). Effect of different cell culture media on the production and glycosylation of a monoclonal antibody from a CHO cell line. Cytotechnology.
- Li, Y. et al. (2025). Urinary extracellular vesicle N-glycomics identifies diagnostic glycosignatures for bladder cancer. Nature Communications.
- Lin, P. et al. (2025). Integrating single-cell and bulk RNA sequencing data to characterize the heterogeneity of glycan-lipid metabolism polarization in hepatocellular carcinoma. Journal of Translational Medicine.
- Liu, S. et al. (2024). Identification of serum N-glycans signatures in three major gastrointestinal cancers by high-throughput N-glycome profiling. Clinical Proteomics.
- MacSharry, J. et al. (2024). Endometriosis specific vaginal microbiota links to urine and serum N-glycome. Scientific Reports.
- Makrydaki, E. et al. (2024). Immobilized enzyme cascade for targeted glycosylation. Nature Chemical Biology.
- Pongracz, T. et al. (2025). GlycoDash: automated, visually assisted curation of glycoproteomics datasets for large sample numbers. Analytical and Bioanalytical Chemistry.
- Renner, T. M. et al. (2025). Modifying the glycosylation profile of SARS-CoV-2 spike-based subunit vaccines alters focusing of the humoral immune response in a mouse model. Communications Medicine.
- Rives, D. et al. (2024). RNASeq highlights ATF6 pathway regulators for CHO cell engineering with different impacts of ATF6β and WFS1 knockdown on fed-batch production of IgG_1. Scientific Reports.
- Shinoda, C. et al. (2025). N-glycan-modified α-L-iduronidase produced by transgenic silkworms ameliorates clinical signs in a Japanese macaque with mucopolysaccharidosis I. Communications Medicine.
- Singh, S. P. et al. (2025). Fe(OTf)3 or Photosensitizer-free blue light activated diazo-thioglycoside donors for Iterative and stereoselective glycosylations. Nature Communications.
- Switala, L. et al. (2024). Engineered nanoparticles promote cardiac tropism of AAV vectors. Journal of Nanobiotechnology.
- Tian, M. et al. (2025). Glycosylation as an intricate post-translational modification process takes part in glycoproteins related immunity. Cell Communication and Signaling.
- Vinicki, M. et al. (2024). Effects of testosterone and metformin on the GlycanAge index of biological age and the composition of the IgG glycome. GeroScience.
- Wan, C. et al. (2024). Cellulose functionalized magnetic beads for high throughput glycosylation analysis in biotherapeutic modalities. Scientific Reports.
- Wang, M. et al. (2024). Spatial omics-based machine learning algorithms for the early detection of hepatocellular carcinoma. Communications Medicine.
- Wang, R. et al. (2025). Antibody–Drug Conjugates (ADCs): current and future biopharmaceuticals. Journal of Hematology & Oncology.
- Xu, T. et al. (2025). Site-specific immunoglobulin G N-glycosylation is associated with gastric cancer progression. BMC Cancer.
- Xu, X. et al. (2025). Profiling of IgG N-glycosylation for axial spondyloarthritis and other rheumatic diseases. Arthritis Research & Therapy.
- Zhang, Q. et al. (2025). Streamlined gram-scale natural N-glycan production using reversible tagging after oxidative release of natural glycans. Communications Chemistry.
- Zheng, Q. et al. (2024). Glycomic profiling of parathyroid neoplasms via lectin microarray analysis. Endocrine.