Sonogenetics: Controlling Cells with Sound

Sonogenetics represents a rapidly emerging field focused on controlling cellular activity, particularly gene expression and neuronal firing, using sound waves—typically in the ultrasound range. Analogous to optogenetics, which uses light, sonogenetics offers the potential for non-invasive control with deeper tissue penetration, overcoming limitations associated with light scattering and absorption in biological tissues.
This approach leverages the physical forces exerted by sound waves—such as radiation force, acoustic streaming, and cavitation—or the thermal energy generated upon ultrasound absorption to interact with cellular components. By engineering cells to respond to these stimuli or by targeting endogenous mechanosensitive pathways, sonogenetics aims to provide precise spatiotemporal control over biological processes for research and therapeutic applications, bridging mechanobiology, synthetic biology, and bioacoustics.
Mechanisms of Sonogenetic Control
The ability to control cells with sound relies on converting acoustic energy into a biological response. Several mechanisms are being explored, including mechanosensitive ion channels, thermal gating, nanoparticle-mediated transduction, and sonoporation.
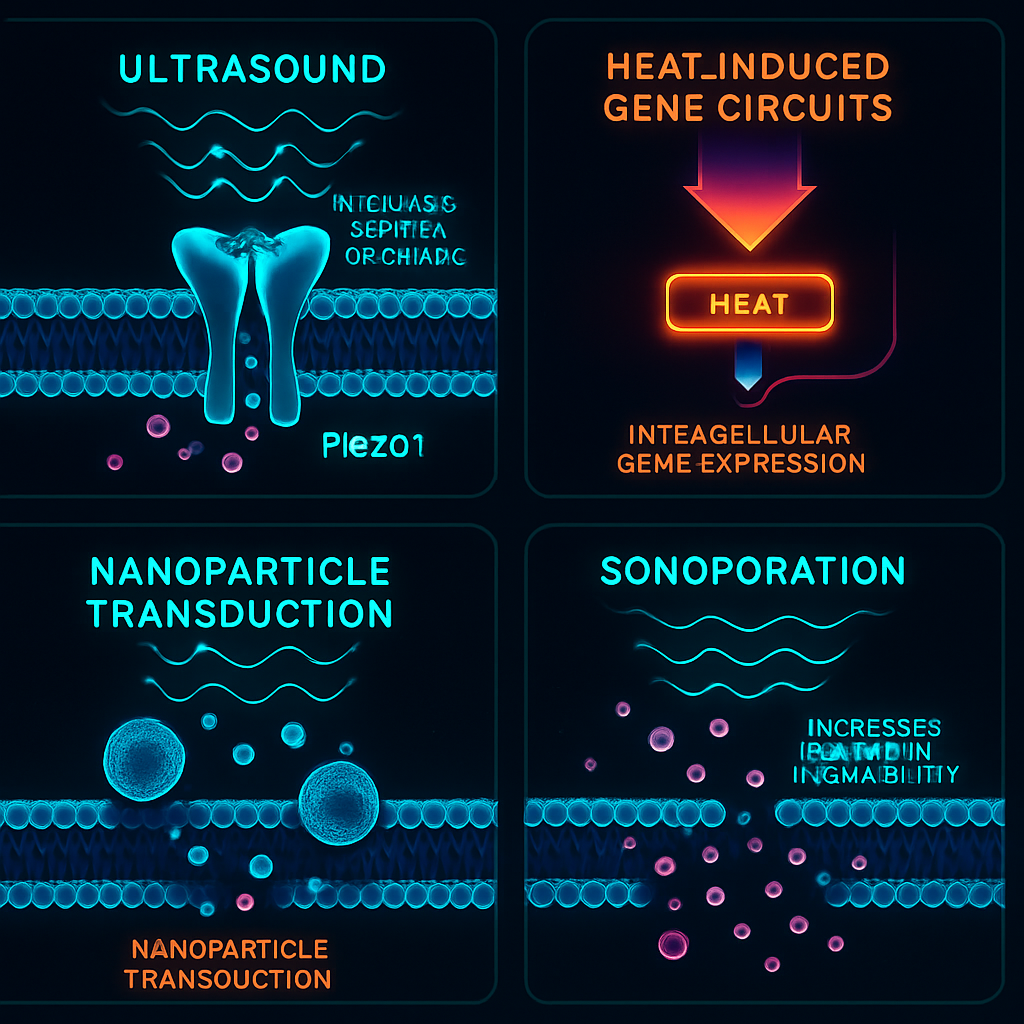
Activation of mechanosensitive ion channels (MSCs) is a primary mechanism, where ultrasound exerts physical force on the cell membrane, triggering endogenous MSCs such as Piezo1 and K2P channels (TRAAK, TREK-1, TREK-2), resulting in altered ion flux and cellular signaling. Cells can be genetically engineered to express specific MSCs, enhancing their sensitivity to sound and enabling control in otherwise unresponsive cell types. The biophysics involve membrane tension, lipid displacement, and cytoskeletal redistribution.
Other mechanisms include focused ultrasound-generated local heating, which activates genetic circuits with heat-sensitive promoters, enabling inducible gene expression. Nanoparticles offer another transduction avenue, converting acoustic energy into force, heat, or reactive oxygen species, thus triggering intracellular pathways. Sonoporation, often assisted by microbubbles, transiently increases membrane permeability, supporting delivery or activation of genetic or pharmacological elements.
Applications and Potential
Sonogenetics holds promise across several domains, including neuromodulation, cell-based therapy, tissue engineering, and basic research.
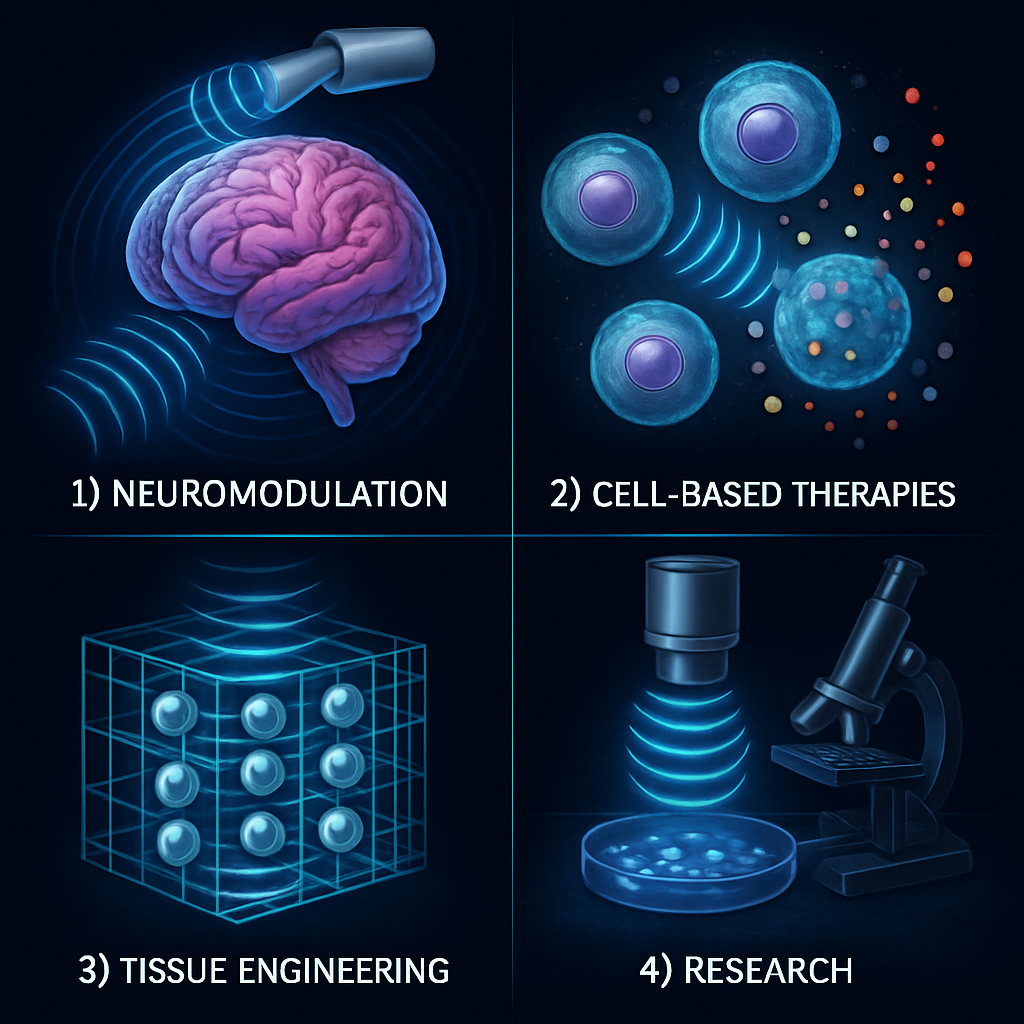
Non-invasive neuromodulation using low-intensity focused ultrasound is being explored for modulation of activity in deep brain regions, offering opportunities for therapies in neurological and psychiatric disorders such as epilepsy, depression, or PTSD. Cell-based therapeutics leverage engineered mammalian cells that release therapeutic agents upon ultrasound stimulation, allowing spatially and temporally controlled delivery to disease sites. In tissue engineering, acoustofluidic forces arrange cells within scaffolds for complex tissue fabrication, and sonogenetics emerges as a tool to study mechanotransduction pathways under controlled acoustic stimuli.
Challenges and Future Directions
Despite its promise, sonogenetics faces several challenges. Achieving high cell-type and spatial specificity remains more difficult than in optogenetics, as ultrasound can affect all sensitive cells within its focus. Mechanism elucidation remains a key area, as the biophysical details of how ultrasound interacts with cellular components is still under investigation and depends on parameters such as frequency, intensity, and pulse duration.

While ultrasound reaches deep tissues, obtaining high spatial resolution equivalent to optical methods is technologically challenging. Establishing standardized, safe protocols (frequency, intensity, pulse duration, duty cycle) is crucial as is long-term safety validation. Integration with other modalities such as optogenetics, magnetogenetics, or closed-loop feedback systems could yield more precise and sophisticated biological control for future applications.
Conclusion
Sonogenetics is a burgeoning field, offering the exciting prospect of non-invasively controlling cellular functions deep within tissues using sound. Its applications span fundamental research in mechanobiology, therapy for neurological disorders, targeted delivery, and complex tissue engineering. By exploiting endogenous mechanosensitivity or incorporating engineered components, researchers are advancing sophisticated tools for precise cellular control. Addressing specificity, mechanism, resolution, and safety challenges will be vital in translating sonogenetics into impactful clinical and research modalities.
References
- Barksdale, B. R., Enten, L., DeMarco, A., Kline, R., Doss, M. K., Nemeroff, C. B., & Fonzo, G. A. (2025). Low-intensity transcranial focused ultrasound amygdala neuromodulation: a double-blind sham-controlled target engagement study and unblinded single-arm clinical trial. Molecular Psychiatry. https://doi.org/10.1038/s41380-025-03033-w
- Bloks, N. G. C., et al. (2024). Hyper-physiologic mechanical cues... Clinical Epigenetics, 16(1), 31. https://doi.org/10.1186/s13148-024-01676-0
- Ghosh, S., & Nagarajan, L. (2025). Neurostimulation in Childhood Epilepsy. Indian Pediatrics. https://doi.org/10.1007/s13312-025-00063-z
- Ivanovski, F., et al. (2024). Ultrasound-mediated spatial and temporal control of engineered cells in vivo. Nature Communications, 15(1), 6683. https://doi.org/10.1038/s41467-024-51620-2
- Jung, H., Lee, H., Shin, M., & Son, D. (2025). Adhesive bioelectronics for closed-loop therapy. Med-X. https://doi.org/10.1007/s44258-025-00055-5
- Kang, B., et al. (2025). Acoustofluidic bioassembly induced morphogenesis for therapeutic tissue fabrication. Nature Communications, 16(1), 4668. https://doi.org/10.1038/s41467-025-59026-4
- Kim, W.-S., et al. (2023). Magneto-acoustic protein nanostructures for non-invasive imaging of tissue mechanics in vivo. Nature Materials, 22(11), 1409–1418. https://doi.org/10.1038/s41563-023-01688-w
- Latypova, A. A., et al. (2024). Magnetogenetics as a promising tool for controlling cellular signaling pathways. Journal of Nanobiotechnology, 22(1), 412. https://doi.org/10.1186/s12951-024-02616-z
- Lin, Z., et al. (2025). Electromagnetic wireless remote control of mammalian transgene expression. Nature Nanotechnology. https://doi.org/10.1038/s41565-025-01929-w
- Liu, P., et al. (2023). Sonogenetic control of multiplexed genome regulation and base editing. Nature Communications, 14(1), 6871. https://doi.org/10.1038/s41467-023-42249-8
- Mehdizadeh, S., et al. (2025). Exosome-powered neuropharmaceutics: unlocking the blood-brain barrier for next-gen therapies. Journal of Nanobiotechnology, 23(1), 316. https://doi.org/10.1186/s12951-025-03352-8
- Neophytou, C., Stylianopoulos, T., & Mpekris, F. (2025). The synergistic potential of mechanotherapy and sonopermeation to enhance cancer treatment effectiveness. npj Biological Physics and Mechanics, 1(1), 2. https://doi.org/10.1038/s44341-025-00017-3
- Rajendran, A. K., et al. (2023). Trends in mechanobiology guided tissue engineering and tools to study cell-substrate interactions: a brief review. Biomaterials Research, 27(1), 58. https://doi.org/10.1186/s40824-023-00393-8
- Ren, S., et al. (2024). Transcriptomic Alterations in the Hippocampus and Prefrontal Cortex of Rats with Chronic Unpredictable Stress Induced by Low-Intensity Pulsed Ultrasound. Molecular Neurobiology. https://doi.org/10.1007/s12035-024-04656-w
- Sorum, B., et al. (2024). Tension activation of mechanosensitive two-pore domain K+ channels TRAAK, TREK-1, and TREK-2. Nature Communications, 15(1), 2818. https://doi.org/10.1038/s41467-024-47208-5
- Wang, H. J., et al. (2024). Microscale geometrical modulation of PIEZO1 mediated mechanosensing through cytoskeletal redistribution. Nature Communications, 15(1), 5370. https://doi.org/10.1038/s41467-024-49833-6
- Xie, T., et al. (2025). The case for hemispheric lateralization of the human amygdala in fear processing. Molecular Psychiatry. https://doi.org/10.1038/s41380-025-02940-2
- Zhao, S., et al. (2025). Topological acoustofluidics. Nature Materials. https://doi.org/10.1038/s41563-025-02169-y
- Zhong, R., et al. (2025). An acoustofluidic embedding platform for rapid multiphase microparticle injection. Nature Communications, 16(1), 4926. https://doi.org/10.1038/s41467-025-59146-x