Sonocytology: Acoustic Profiling of Cellular Dynamics and Mechanotransduction Pathways
Sonocytology is conceptualized as an emerging interdisciplinary field dedicated to the study of cells using acoustic waves. This builds upon the growing recognition that physical cues, including acoustic vibrations, profoundly influence biological systems from microbes to complex organisms (de la Viuda et al., 2025; Ostasevicius et al., 2024). Sonocytology aims to provide non-invasive, real-time insights into cellular architecture, mechanical properties, dynamic behaviors, and mechanotransduction pathways. By leveraging the unique interactions between sound energy and biological matter at the microscale, it holds the potential to revolutionize our understanding of fundamental cell biology and to foster novel diagnostic and therapeutic strategies. The ability of acoustic waves to penetrate optically opaque media and to exert precise forces offers distinct advantages over existing cytological techniques, paving the way for label-free characterization of cells in their native environments and for probing the intricate ways cells sense and respond to mechanical cues.
The core premise of sonocytology is that the acoustic properties and responses of cells can serve as intrinsic biomarkers of their physiological or pathological state. Variations in cell size, shape, density, elasticity, and intracellular organization can alter how cells interact with sound waves (e.g., scattering, absorption, impedance). Moreover, active cellular responses to acoustic stimuli, mediated by mechanosensitive structures, can reveal functional aspects of cellular mechanobiology. This field, therefore, sits at the intersection of biophysics, acoustic engineering, cell biology, and computational science, aiming to develop novel instrumentation, experimental methodologies, and analytical frameworks to decode the wealth of information contained in cell-sound interactions.
Acoustic Methodologies for Cellular Interrogation
The foundation of sonocytology lies in the development and application of sophisticated acoustic tools capable of interacting with cells at high resolution. Acoustofluidics, merging acoustics and microfluidics, has been pivotal. Techniques like acoustic tweezers utilize standing sound waves to create pressure gradients, enabling precise, non-contact manipulation, sorting, patterning, and aggregation of cells within microfluidic chips (Kang et al., 2025; Surappa et al., 2024). These methods allow for the creation of controlled cellular microenvironments and the study of cell-cell interactions. The versatility of acoustofluidics also extends to enabling rapid multi-phase microparticle injection into aqueous droplets (Zhong et al., 2025), a principle adaptable for controlled delivery of substances to cells or for constructing complex cellular assays. For instance, the acoustofluidic bioassembly approach demonstrates the fabrication of tissues with regulated structures by precise cell arrangement, highlighting the potential for morphogenesis studies (Kang et al., 2025).
Further advancements include the development of topological acoustofluidics, which can create complex acoustic landscapes like valley streaming vortices and chiral swirling patterns for nuanced particle transport and trapping, even at the nanoscale for molecules like DNA (Zhao, S. et al., 2025). The engineering of dynamically reconfigurable acoustofluidic metasurfaces offers even finer control over acoustic fields, enabling subwavelength manipulation and patterning of individual or collective particles and cells (Surappa et al., 2024). Such precise control is crucial for investigating localized cellular responses. While high-frequency ultrasound is established in medical imaging, its adaptation for microscopic cellular profiling, perhaps through acoustic impedance microscopy or by analyzing the acoustic emissions from cells under specific stimuli (drawing inspiration from observations like cavitation near needles, Kiviluoto et al., 2025), could yield novel intrinsic contrast mechanisms reflecting cellular mechanical states. The development of self-focusing high-frequency ultrasonic transducers for other fields (Zhao, J. et al., 2025) indicates a technological pathway for creating such specialized bio-acoustic tools.
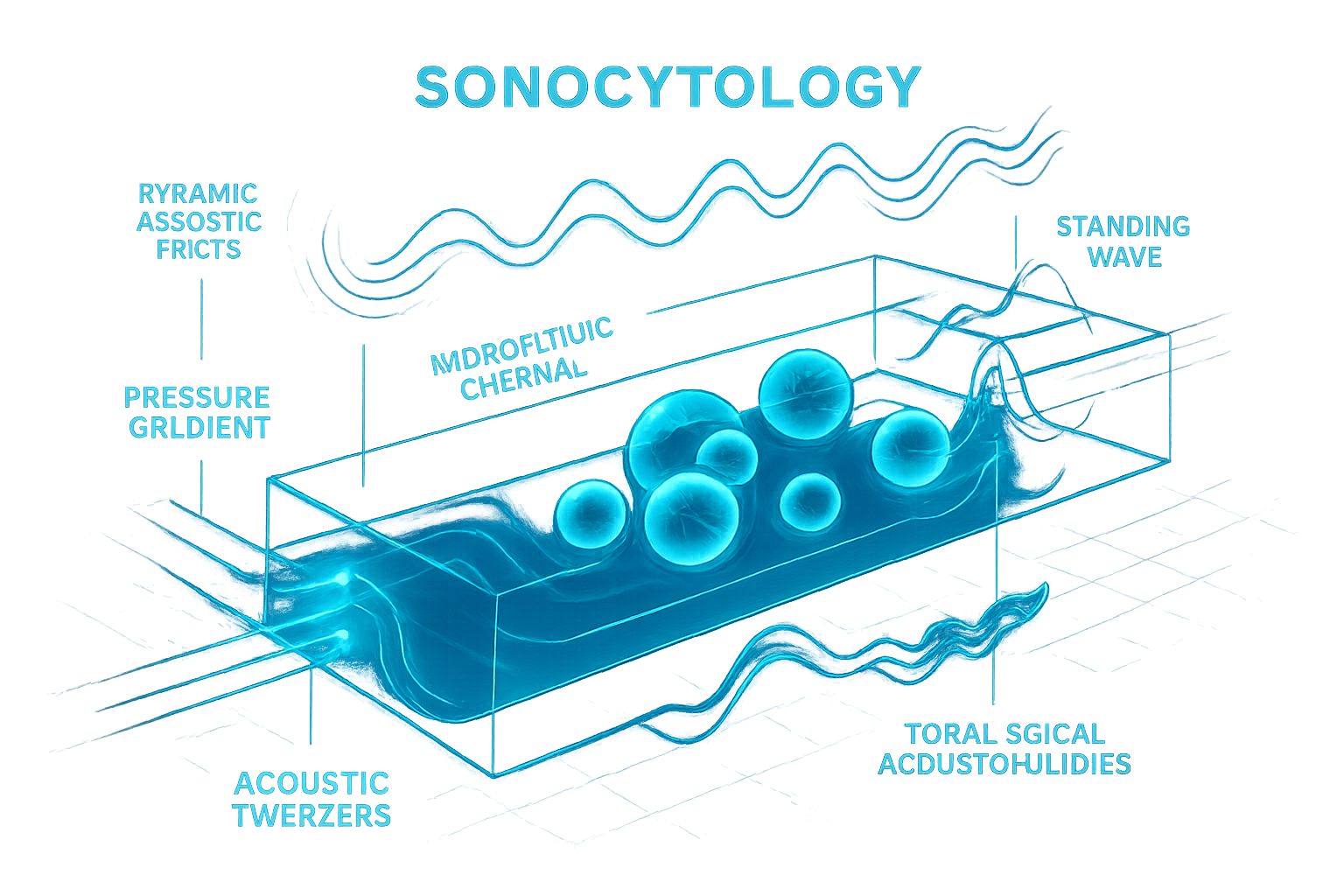
Cellular Responses to Acoustic Perturbation
Cells are inherently mechanosensitive, and acoustic waves, as a form of mechanical energy, can elicit a spectrum of responses. These range from subtle alterations in intracellular dynamics and gene expression to more overt changes in morphology, adhesion, and viability. Investigations have shown that audible acoustic waves (440 Hz, 14 kHz) can modulate gene expression in myoblasts, with Ptgs2/Cox-2 identified as a key immediate sound-responding gene, its activation being dependent on focal adhesion kinase (Kumeta et al., 2025). This suggests that even low-intensity sound can trigger specific signaling cascades. The study by Kumeta et al. (2025) further demonstrated that adipocyte differentiation can be significantly suppressed by acoustic stimulation, opening avenues for sound-based control of cell fate.
The mechanical properties of cells, such as stiffness and viscoelasticity, are critical indicators of cellular health and function. The quest to measure these properties is a significant driver in mechanobiology, with various advanced techniques emerging to probe intracellular mechanics (Molnar & Manneville, 2025; Català-Castro et al., 2024; Mukherjee et al., 2024). Sonocytology aims to develop acoustic counterparts to these techniques, potentially using focused ultrasound to apply minute forces and measure cellular deformation, or analyzing the acoustic scattering signatures of cells to infer their mechanical phenotype. The observation that cellular contractile forces and membrane biophysics are altered in cancer (Alqabandi et al., 2024) suggests that acoustic profiling could detect such pathological changes. The field will explore how different acoustic frequencies and intensities can be used to map these mechanical properties with spatial and temporal resolution, potentially revealing localized changes within cellular compartments or during dynamic processes like cell division or migration.
Mechanotransduction: From Sound Waves to Biological Signals
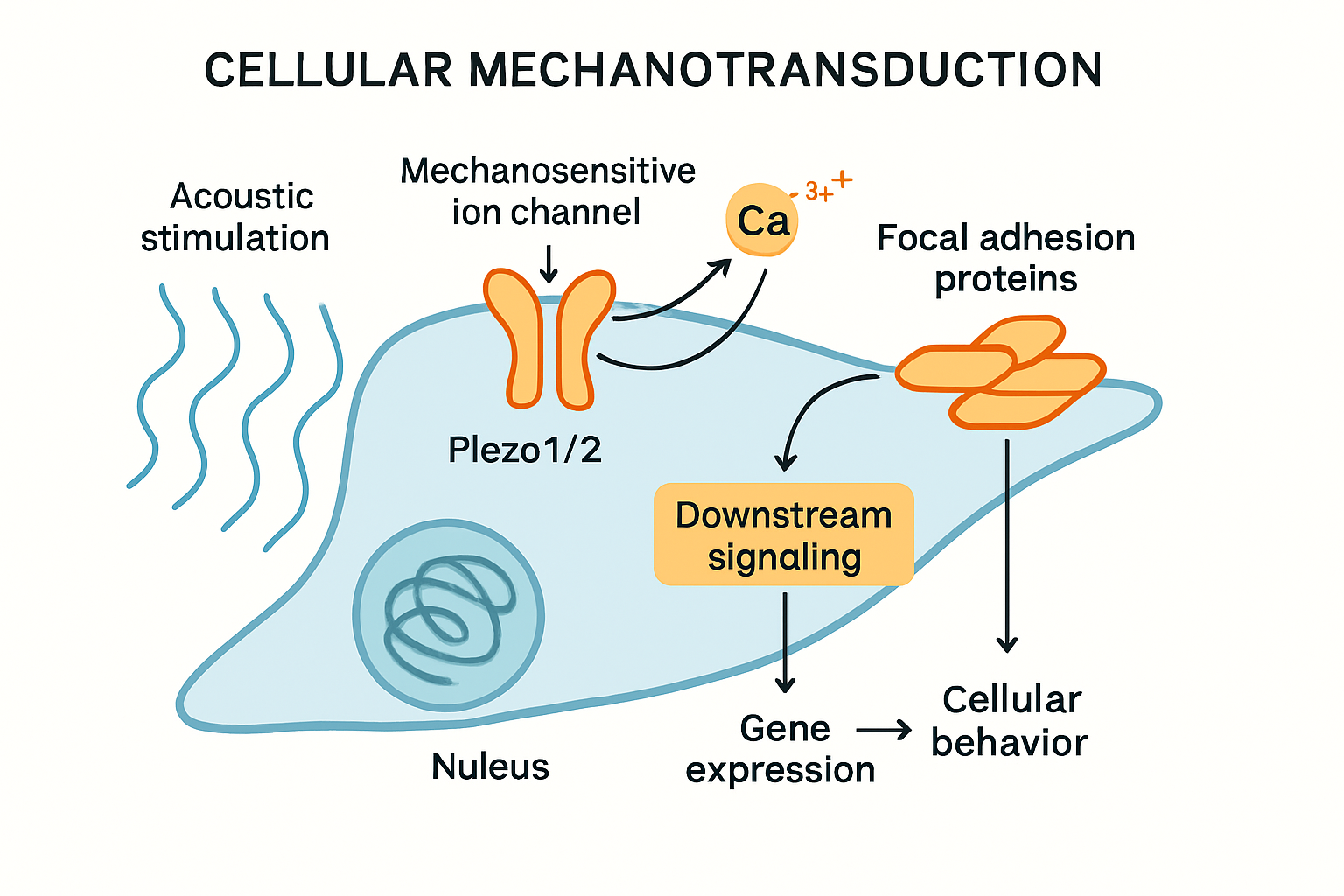
A central tenet of sonocytology is the elucidation of mechanotransduction pathways activated by acoustic stimuli. Mechanosensitive ion channels, such as Piezo1 and Piezo2, are prime candidates for sensing acoustic forces. These channels are known to respond to various mechanical cues, including membrane stretch and shear stress, converting them into electrical and chemical signals, primarily through calcium influx. Studies have implicated Piezo1 in diverse physiological and pathological processes, including sepsis-induced myocardial dysfunction (Gong et al., 2025) and its expression is altered in cancers (Liang et al., 2025), suggesting its role as a key transducer of mechanical information. The identification of Piezo channels as putative baroreceptors further underscores their sensitivity to pressure changes (Yundung et al., 2025).
Sonocytology will investigate how specific acoustic parameters (frequency, intensity, waveform) selectively activate these and other mechanosensors like integrins, transmembrane receptors, and the cytoskeleton itself. The ensuing intracellular signaling—involving calcium oscillations, activation of protein kinases (e.g., FAK, Src), Rho-GTPase signaling, and cytoskeletal remodeling—ultimately dictates the cellular response, be it adaptive or pathological. Understanding these pathways is crucial for interpreting acoustic profiling data and for designing targeted "sonogenetic" interventions where sound is used to precisely control cellular activity through defined molecular transducers. The field will explore whether cells have unique "acoustic signatures" based on the activity, localization, and expression patterns of their mechanotransduction machinery, and how these signatures change in response to environmental cues or during disease progression.
Applications and Future Perspectives in Sonocytology
The implications of sonocytology are far-reaching. Diagnostically, acoustic profiling could offer rapid, label-free characterization of cells, enabling the detection of disease states associated with altered mechanical properties or mechanosensitive responses, such as cancer, fibrosis, or neurodegenerative conditions. For instance, variations in Piezo1/2 expression or activity, detectable through their response to specific acoustic stimuli, could serve as highly sensitive biomarkers (Liang et al., 2025). The ability to perform such analyses in situ, perhaps even distinguish circulating tumor cells based on their acoustic phenotype, would be a significant advance. Therapeutically, focused ultrasound is already used for tissue ablation and to enhance drug delivery (sonoporation). Sonocytology could refine these approaches, allowing for targeted modulation of cellular functions at a finer scale, for instance, using low-intensity pulsed ultrasound (LIPUS)-like modalities (Mahmoud et al., 2025) to promote tissue regeneration by selectively stimulating beneficial cellular pathways or guiding cell differentiation as hinted by Kumeta et al. (2025).
Future research will focus on developing higher-resolution and higher-throughput acoustic systems, potentially integrating them with advanced optical microscopy (e.g., light-sheet, super-resolution) and multi-omics analyses for comprehensive single-cell profiling. A major challenge lies in deciphering the complex interplay between acoustic forces and the cellular machinery across multiple scales, from molecular interactions to tissue-level organization. This involves not only identifying the primary mechanosensors but also mapping the downstream signaling networks, epigenetic modifications, and gene regulatory changes. The development of sophisticated computational models to simulate cell-sound interactions and predict cellular responses will be crucial. Furthermore, the translation of sonocytology into clinical practice will require robust, user-friendly instrumentation and standardized protocols, along with rigorous validation against existing gold standards.
Conclusion
Sonocytology, as proposed here, represents a convergence of acoustics, biophysics, and cell biology, aiming to utilize sound as a precise tool for interrogating and modulating cellular behavior. By non-invasively probing cellular mechanics, dynamics, and the intricate pathways of mechanotransduction, this field offers the prospect of novel diagnostic markers derived from a cell's "acoustic fingerprint" and innovative therapeutic strategies based on targeted acoustic stimulation. The continued development of advanced acoustic manipulation and sensing technologies, coupled with a deeper understanding of how cells perceive and respond to sound, will be instrumental in realizing the full potential of sonocytology. This endeavor could significantly impact areas from fundamental biological research, such as understanding morphogenesis, immune cell function, and intercellular communication, to clinical applications like early disease detection, personalized medicine, and regenerative therapies. The exploration of the "sonome" – the complete set of acoustic interactions and responses of a cell – is a rich territory for future scientific inquiry, promising to reveal new layers of biological complexity and control.
References
- Alqabandi, J. A. et al. (2024). An innovative cellular medicine approach via the utilization of novel nanotechnology-based biomechatronic platforms as a label-free biomarker for early melanoma diagnosis. Scientific Reports. https://doi.org/10.1038/s41598-024-79154-z
- Català-Castro, F. et al. (2024). Measuring age-dependent viscoelasticity of organelles, cells and organisms with time-shared optical tweezer microrheology. Nature Nanotechnology. https://doi.org/10.1038/s41565-024-01830-y
- de la Viuda, V. et al. (2025). Physical communication pathways in bacteria: an extra layer to quorum sensing. Biophysical Reviews. https://doi.org/10.1007/s12551-025-01290-1
- Gong, A. et al. (2025). Piezo1 activation protects against sepsis-induced myocardial dysfunction in a pilot study. Scientific Reports. https://doi.org/10.1038/s41598-025-00829-2
- Kang, B. et al. (2025). Acoustofluidic bioassembly induced morphogenesis for therapeutic tissue fabrication. Nature Communications. https://doi.org/10.1038/s41467-025-59026-4
- Kiviluoto, J. et al. (2025). Cavitation activity induced by spring-loaded core needle biopsy devices. Scientific Reports. https://doi.org/10.1038/s41598-025-97497-z
- Kumeta, M. et al. (2025). Acoustic modulation of mechanosensitive genes and adipocyte differentiation. Communications Biology. https://doi.org/10.1038/s42003-025-07969-1
- Liang, T. et al. (2025). Comprehensive analysis of mRNA expression of Piezo1 and Piezo2 in tumor samples and their prognostic implications in gastric cancer. Discover Oncology. https://doi.org/10.1007/s12672-025-02309-5
- Mahmoud, E. S. et al. (2025). Low intensity pulsed ultrasound versus low-level laser therapy on peri-implant marginal bone preservation and soft tissue healing following dental implant surgery: a randomized controlled trial. Head & Face Medicine. https://doi.org/10.1186/s13005-025-00502-z
- Molnar, K., & Manneville, J.-B. (2025). Emerging mechanobiology techniques to probe intracellular mechanics. npj Biological Physics and Mechanics. https://doi.org/10.1038/s44341-025-00016-4
- Mukherjee, S. et al. (2024). Anisotropy, topography and non-newtonian properties of cellular interiors probed by helical magnetic nanobots. Journal of Micro and Bio Robotics. https://doi.org/10.1007/s12213-024-00176-x
- Ostasevicius, V. et al. (2024). Vibrational Activation of Blood Flow. In: Noninvasive Therapeutic Technologies. https://doi.org/10.1007/978-3-031-79025-6_3
- Surappa, S. et al. (2024). Dynamically reconfigurable acoustofluidic metasurface for subwavelength particle manipulation and assembly. Nature Communications. https://doi.org/10.1038/s41467-024-55337-0
- Yundung, Y. et al. (2025). Identification of putative baroreceptors in human aortic arch by histological and omics analyses. Hypertension Research. https://doi.org/10.1038/s41440-025-02217-9
- Zhao, J. et al. (2025). Self-focusing high-frequency ultrasonic transducers for non-destructive testing applications. Scientific Reports. https://doi.org/10.1038/s41598-025-93195-y
- Zhao, S. et al. (2025). Topological acoustofluidics. Nature Materials. https://doi.org/10.1038/s41563-025-02169-y
- Zhong, R. et al. (2025). An acoustofluidic embedding platform for rapid multiphase microparticle injection. Nature Communications. http://dx.doi.org/10.1038/s41467-025-59146-x