Sonocatalytic Degradation of Per- and Polyfluoroalkyl Substances (PFAS): Leveraging Acoustic Cavitation to Break Strong Carbon-Fluorine Bonds in Environmental Remediation

Per- and polyfluoroalkyl substances (PFAS) represent a class of thousands of synthetic chemicals characterized by chains of carbon atoms saturated with fluorine atoms. The carbon-fluorine (C-F) bond is one of the strongest in organic chemistry, granting these compounds exceptional thermal and chemical stability. This has made them invaluable in a vast range of industrial and consumer products, from non-stick cookware to firefighting foams. However, this same stability renders them extraordinarily persistent in the environment, earning them the moniker "forever chemicals." Their widespread contamination of water resources, coupled with evidence of bioaccumulation and toxicity, presents a formidable global environmental and health challenge. Traditional water treatment methods, such as activated carbon adsorption, merely transfer the pollutants from one phase to another without destroying them. Sonocatalytic degradation is emerging as a powerful and promising destructive technology. By applying high-frequency ultrasound to an aqueous solution containing a catalyst, this advanced oxidation process (AOP) generates localized pockets of extreme temperature and pressure, creating conditions sufficient to sever the resilient C-F bond and mineralize these persistent pollutants.
The Physics of Destruction: Acoustic Cavitation
The fundamental mechanism underpinning the sonocatalytic degradation of PFAS is acoustic cavitation. When high-frequency ultrasound (typically >20 kHz) propagates through a liquid, it creates cycles of compression and rarefaction. During the low-pressure rarefaction phase, microscopic bubbles, or cavities, form and grow. During the subsequent high-pressure compression phase, these bubbles are forced to collapse violently. This implosion is not uniform; it generates transient, localized ‘hotspots’ with temperatures reaching several thousand Kelvin and pressures exceeding hundreds of atmospheres. These extreme conditions create a unique physicochemical environment. Firstly, molecules trapped within or near the collapsing bubble undergo pyrolysis, or thermal decomposition. Secondly, the intense energy cleaves water molecules (H₂O) into highly reactive hydroxyl (•OH) and hydrogen (•H) radicals. The combination of direct thermal breakdown and attack by potent radicals provides the necessary force to break the C-F bonds that define PFAS molecules, initiating their degradation cascade.

Enhancing Efficiency: The Role of Sonocatalysts
While sonolysis (the application of ultrasound alone) can break down PFAS, the efficiency is often limited by the rate of bubble nucleation and the availability of reactive species. Sonocatalysis dramatically enhances the process by introducing solid catalysts into the system. These materials serve a dual purpose. Firstly, the microscopic imperfections and large surface area of the catalyst particles act as nucleation sites, promoting the formation of more cavitation bubbles and thus increasing the number of reactive hotspots throughout the solution. This distribution of energy overcomes a key limitation of sonolysis alone. Secondly, the catalyst surface itself becomes an active participant in the degradation process. The immense energy released during bubble collapse can excite the catalyst material, generating additional reactive oxygen species (ROS) and promoting charge carrier separation, which accelerates the oxidation of adsorbed PFAS molecules. Research into catalysts for other AOPs, such as doped metal oxides like ZnO or piezoelectric composites like BaTiO₃/WS₂ (Fazli et al., 2022; Rodríguez-Flores et al., 2024), provides a blueprint for designing catalysts that can synergistically enhance the destructive power of cavitation for PFAS remediation.
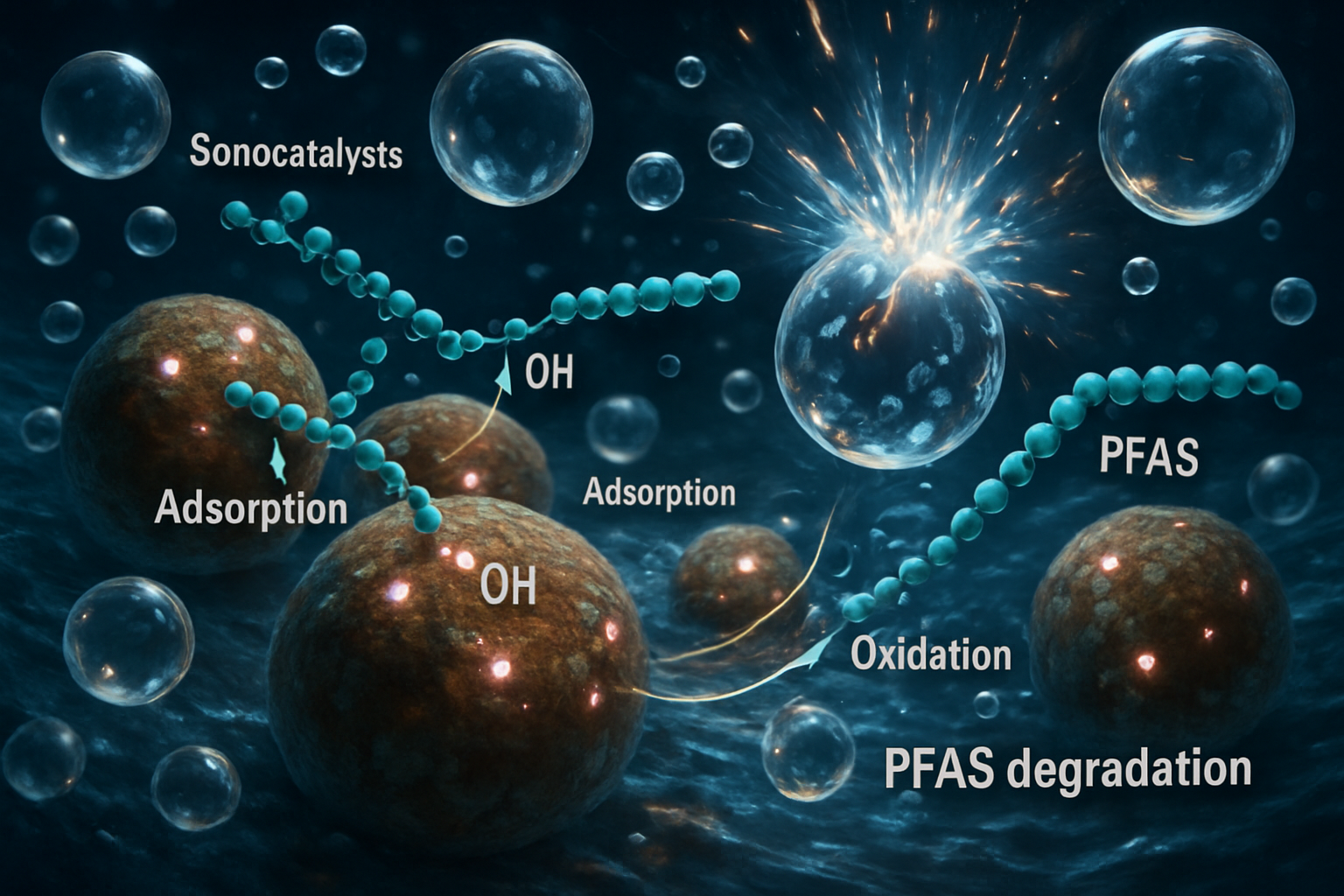
Breaking the Unbreakable: Degradation Pathways and Mechanisms
The sonocatalytic destruction of PFAS is not a single event but a multi-step process. The primary and most critical step is the cleavage of the molecule, which is thought to occur at the bubble-water interface where hydrophobic PFAS molecules accumulate. For long-chain PFAS like perfluorooctanoic acid (PFOA), the initial attack is believed to involve two main pathways. The first is pyrolytic cleavage of the terminal carboxylate or sulfonate headgroup, which is thermally less stable than the fluorinated carbon chain. The second is attack by hydroxyl radicals on this same hydrophilic headgroup. Once the headgroup is removed, the decapitated hydrophobic chain undergoes stepwise "unzipping," where CF₂ units are cleaved one by one. This process is analogous to degradation patterns seen in other AOPs, such as electrochemical oxidation, where PFOA breaks down into shorter-chain perfluorinated carboxylic acids (Trzcinski & Harada, 2024). The ultimate goal of this process is complete mineralization, converting the organic fluorine into inorganic fluoride ions (F⁻), which is a definitive measure of successful destruction.
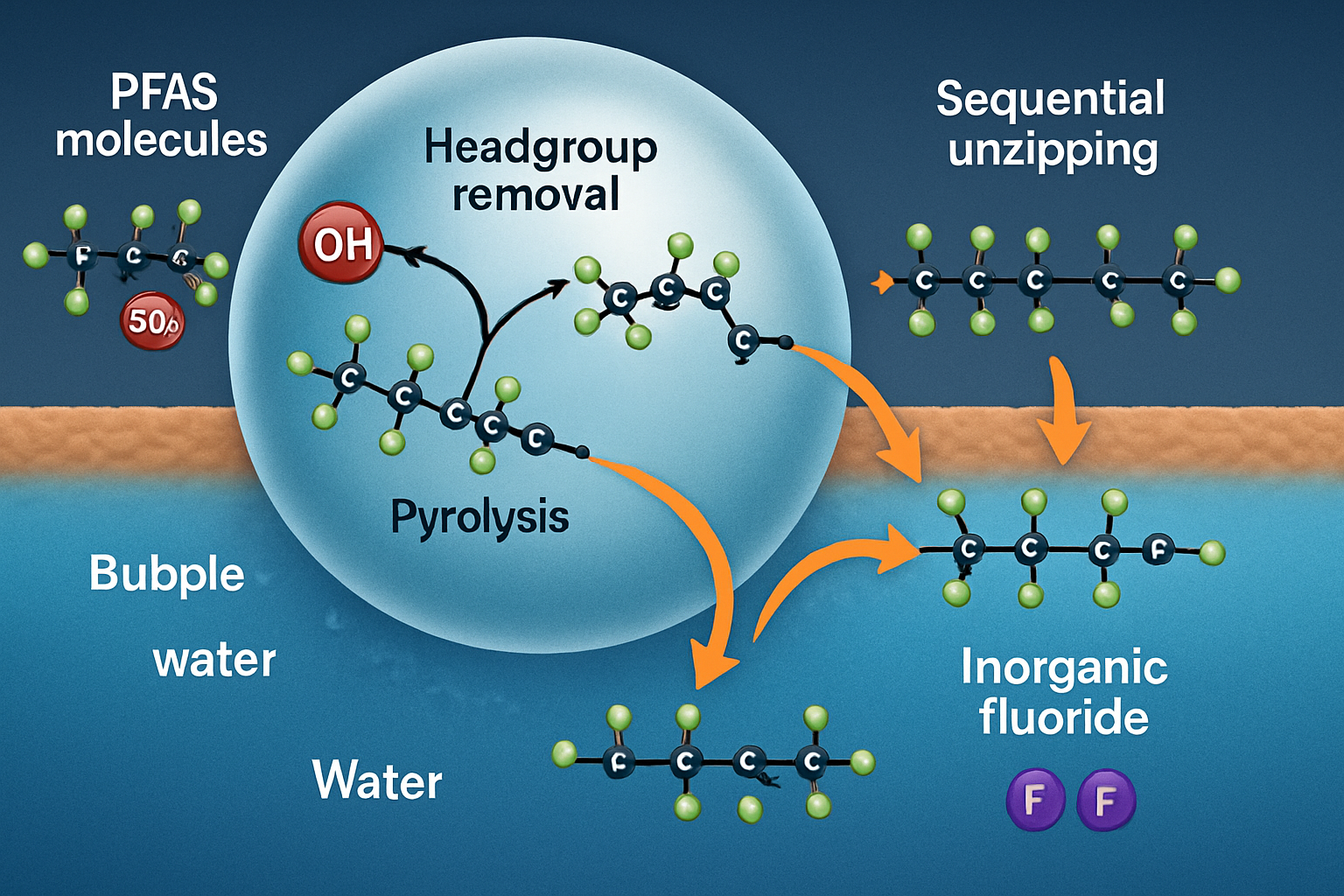
Conclusion
Sonocatalysis stands out as one of the few technologies capable of destructively eliminating PFAS, addressing the root of the contamination problem rather than simply managing it. By transforming acoustic energy into localized chemical reactors, it can overcome the immense strength of the C-F bond. However, significant challenges, particularly high energy consumption and the potential interference from complex environmental matrices, have hindered its large-scale application (Tshangana et al., 2025). The future of effective PFAS remediation likely lies not in a single technology, but in integrated treatment trains. A highly speculative but promising approach would involve a two-stage system: first, a pre-concentration step using high-affinity adsorbents (Mian et al., 2025) to pull PFAS from large volumes of contaminated water into a smaller, concentrated volume. This concentrate would then be treated by an energy-efficient sonocatalytic reactor. This strategy would make the high energy cost of sonocatalysis more feasible by focusing its power where it is most needed. Further innovation in catalyst design, such as developing bifunctional piezo-photocatalysts, and reactor engineering to optimize mass transfer will be crucial to realizing the full potential of this technology and providing a definitive solution to the global challenge of "forever chemicals."
References
- Fazli, A., Zakeri, F., Khataee, A., & Orooji, Y. (2022). A BaTiO3/WS2 composite for piezo-photocatalytic persulfate activation and ofloxacin degradation. Communications Chemistry. https://doi.org/10.1038/s42004-022-00707-2
- Mian, M. M., Zhu, J., Jiang, X., & Deng, S. (2025). Recent advances in activated carbon driven PFAS removal: structure-adsorption relationship and new adsorption mechanisms. Frontiers of Environmental Science & Engineering. https://doi.org/10.1007/s11783-025-1998-3
- Riegel, M., Haist-Gulde, B., & Sacher, F. (2023). Sorptive removal of short-chain perfluoroalkyl substances (PFAS) during drinking water treatment using activated carbon and anion exchanger. Environmental Sciences Europe. https://doi.org/10.1186/s12302-023-00716-5
- Rodríguez-Flores, T., Hernández-Pérez, I., Huerta-Hernández, G. E., Suárez-Parra, R., & Haro-Pérez, C. (2024). Sonocatalytic degradation of RB-5 dye using ZnO nanoparticles doped with transition metals. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-024-35776-4
- Trzcinski, A. P., & Harada, K. (2024). Combined adsorption and electrochemical oxidation of perfluorooctanoic acid (PFOA) using graphite intercalated compound. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-024-32449-0
- Tshangana, C. S., Nhlengethwa, S. T., Glass, S., Denison, S., Kuvarega, A. T., Nkambule, T. T. I., Mamba, B. B., Alvarez, P. J. J., & Muleja, A. A. (2025). Technology status to treat PFAS-contaminated water and limiting factors for their effective full-scale application. npj Clean Water. https://doi.org/10.1038/s41545-025-00457-3