Radiolytic Degradation of Pharmaceuticals in Deep Space: Developing Novel Excipients and On-Demand Synthesis Platforms to Ensure Crew Health on Long-Duration Missions
As humanity stands on the precipice of becoming a multi-planetary species, with crewed missions to Mars and beyond transitioning from science fiction to concrete engineering roadmaps, we must confront the immense challenge of ensuring human health far from Earth. A three-year mission to Mars will be the longest and most isolated human endeavor in history, demanding complete medical self-sufficiency. A critical and under-appreciated component of this is the stability of the onboard pharmacy. Pharmaceuticals are susceptible to degradation from the harsh deep space environment, particularly the constant bombardment of Galactic Cosmic Radiation (GCR). The prevailing assumption has been that GCR will cause significant radiolytic degradation, breaking down active pharmaceutical ingredients (APIs) and rendering medications ineffective or even toxic over time. This presents a mission-critical risk, as resupply is impossible.
However, recent evidence has introduced a crucial nuance to this problem, suggesting that for certain drug formulations, the threat may be less severe than once feared. This emerging complexity calls for a sophisticated, dual-pronged strategy. It is no longer a simple matter of shielding medicines; instead, the future of deep space medicine will likely depend on two parallel and complementary development paths. The first involves the near-term, targeted development of novel, radioprotective excipients—inactive ingredients that actively shield the API from radiation—inspired by the resilient chemistry of prebiotic life. The second, a longer-term paradigm shift, focuses on creating robust, automated on-demand synthesis platforms capable of manufacturing complex or unstable medications in-situ, using local resources and eliminating the need for long-term storage altogether.
Re-evaluating the Threat: The Critical Role of Formulation
The primary mechanism of radiation damage to pharmaceuticals is radiolysis, which occurs via two pathways: direct ionization of the drug molecule, and, more significantly, the indirect effect where radiation splits surrounding molecules (especially water) into highly reactive free radicals, such as reactive oxygen species (ROS). These ROS then attack and degrade the API. For this reason, liquid and semi-solid formulations have long been considered exceptionally vulnerable. The long-held paradigm was that GCR would be a universal "showstopper" for maintaining a long-term pharmacy in space.
However, a landmark study by Plante et al. (2025) has challenged this view. By exposing solid oral formulations of common medications like Ibuprofen and Amoxicillin to simulated GCR, they found no significant acceleration in degradation compared to ground controls over a three-year period. This suggests that the molecular environment immediately surrounding the API is paramount. In a well-formulated, low-water solid tablet, the pathways for indirect radiolytic damage are severely restricted. This finding does not eliminate the threat, but rather reframes it: the problem may not be radiation in isolation, but radiation acting on specific formulations. This is further complicated by findings from Dormán et al. (2024), whose stability studies of Remdesivir on the ISS yielded inconsistent results between missions, highlighting the complex interplay of variables in the true space environment. The critical insight is that formulation is not a passive carrier but the first line of defense against radiolytic decay.

Inspired by Prebiotic Life: A New Frontier for Radioprotective Excipients
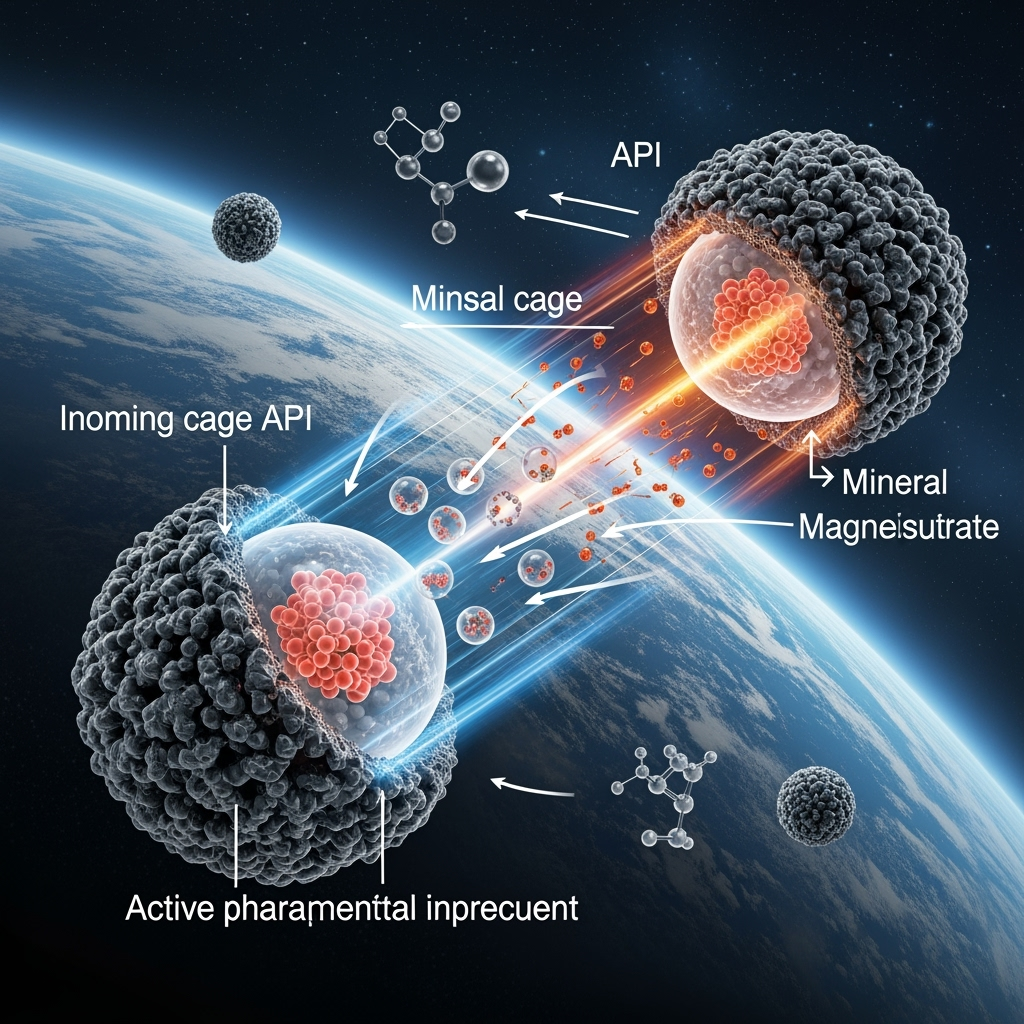
If formulation is the key, then the next frontier is designing active formulations. This requires moving beyond simple binders and fillers to intelligent excipients designed explicitly for radioprotection. A remarkable source of inspiration for such materials comes from a seemingly unrelated field: astrobiology and the study of the origin of life. Research into how the first biomolecules survived the intense radiation environment of the early Earth has revealed powerful protective mechanisms. A study by Dai et al. (2023) demonstrated the creation of a radioresistant protocell model where inorganic polyphosphate-manganese complexes formed a protective shell that effectively scavenged ROS and shielded internal proteins and DNA.
This provides a direct conceptual blueprint for a new class of pharmaceutical excipients. One can envision creating formulations where the API is micro-encapsulated within a "mineral cage" of manganese, magnesium sulfate, or other inorganic compounds. These cages would function in two ways: providing a physical barrier to direct GCR hits and, more importantly, chemically neutralizing the free radicals generated in the residual matrix before they can reach and damage the API. Supporting this concept, work by Alberini et al. (2024) has shown that magnesium sulfate can effectively photoprotect organic molecules from UV radiation on the Martian surface. By borrowing these principles from nature's most radiation-hardened systems, we can engineer exceptionally stable solid-dose medications specifically for the deep space environment, securing our supply of essential, first-line drugs.
The In-Situ Apothecary: On-Demand Pharmaceutical Synthesis
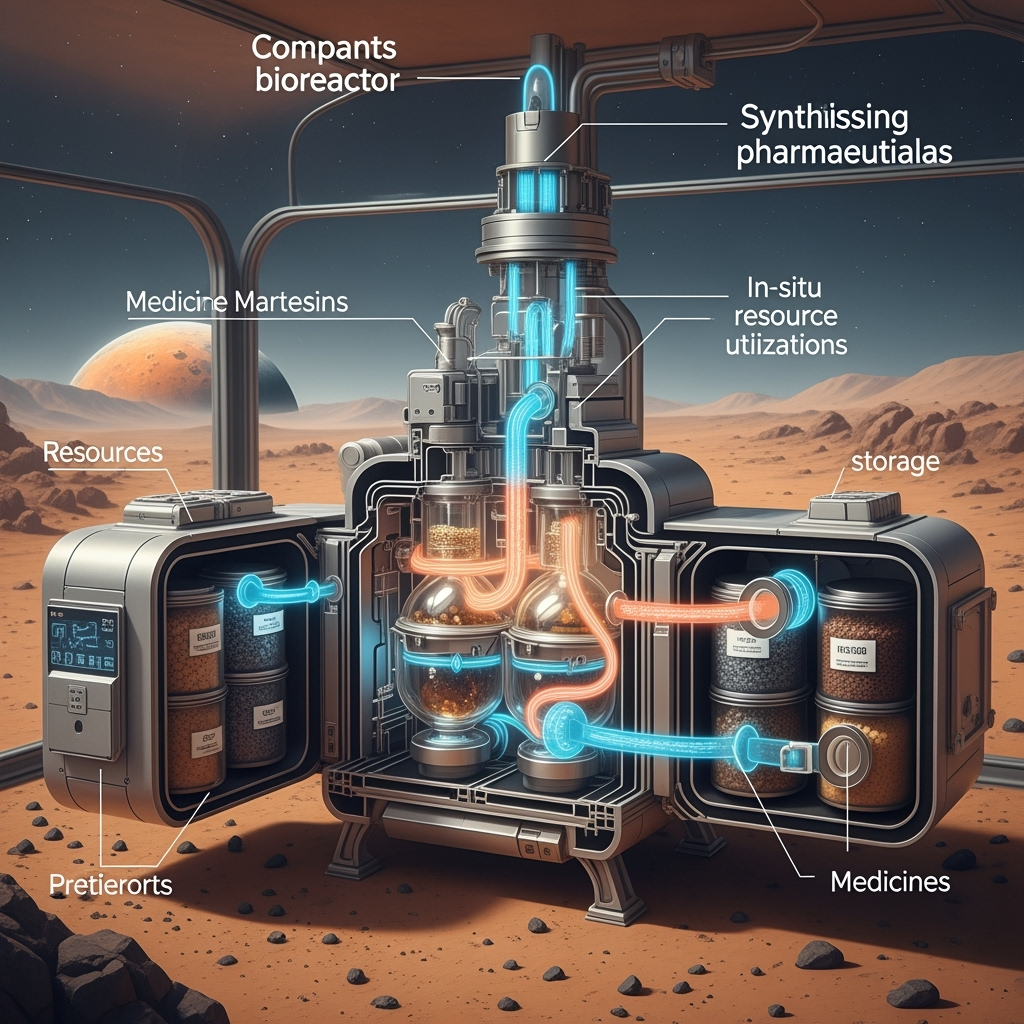
While advanced excipients can protect many small-molecule drugs, this strategy is insufficient for all medical needs. Many modern therapeutics, including peptides, antibodies, and other biologics, are inherently fragile and difficult to stabilize even on Earth. Furthermore, as shown by Diaz et al. (2024), a significant portion of the current ISS formulary has a terrestrial shelf-life of less than three years, meaning they would expire during a Mars mission regardless of radiation. The solution for these substances, and for responding to unforeseen medical emergencies, is to bypass storage entirely through on-demand synthesis.
This concept, once relegated to theory, is rapidly becoming a technological reality. Researchers are now developing and testing compact, automated bioreactors for space. Lee et al. (2025) have demonstrated an "alternative feedstock-driven in-situ biomanufacturing" (AF-ISM) process that uses Martian regolith simulant, plastic waste, and human waste to cultivate engineered microbes that produce valuable compounds. This aligns perfectly with the principle of in-situ resource utilization (ISRU). Complementing the chemical process, Liu et al. (2025) have successfully flight-tested a modular, autonomous bioreactor on the ISS, proving the viability of the hardware. The "in-situ apothecary" would be a versatile instrument capable of synthesizing a wide range of complex drugs from stable precursors or recycled waste streams, providing the ultimate in medical capability and mission flexibility. The challenge now shifts from storage to logistics, requiring careful management of precursor chemicals and biological feedstocks, as modeled by Yates et al. (2024), who highlight the importance of accounting for elements like nitrogen in a closed-loop system.
Conclusion
The threat of radiolytic degradation to pharmaceuticals is not a simple, monolithic obstacle but a complex, formulation-dependent challenge. The path to ensuring crew health on long-duration missions is not a single solution but a pragmatic, dual-pronged strategy. For the core set of predictable, essential small-molecule drugs, we must pursue the development of advanced radioprotective formulations, drawing inspiration from the robust chemistry that allowed life to begin in harsh environments. Simultaneously, for complex, unstable, or contingently needed medicines, we must accelerate the development of automated, on-demand synthesis platforms that transform the concept of a pharmacy from a cabinet of pills into a versatile chemical and biological production tool. This hybrid approach—a small formulary of hyper-stabilized drugs complemented by an in-situ apothecary—will provide the resilient and adaptable medical capability required for humanity to safely take its next giant leap into the cosmos. The research spurred by this grand challenge will not only benefit future astronauts but promises innovations in drug stability and point-of-care manufacturing for remote and underserved locations on Earth.
References
- Alberini, A., Fornaro, T., García-Florentino, C., et al. (2024). Investigating the stability of aromatic carboxylic acids in hydrated magnesium sulfate under UV irradiation to assist detection of organics on Mars. Scientific Reports. https://doi.org/10.1038/s41598-024-66669-8
- Dai, S., Xie, Z., Wang, B., et al. (2023). An inorganic mineral-based protocell with prebiotic radiation fitness. Nature Communications. https://doi.org/10.1038/s41467-023-43272-5
- Diaz, T. E., Ives, E. C., Lazare, D. I., & Buckland, D. M. (2024). Expiration analysis of the International Space Station formulary for exploration mission planning. npj Microgravity. https://doi.org/10.1038/s41526-024-00414-3
- Dormán, G., Buchholcz, B., Puskás, I., et al. (2024). Repetitive stability study of remdesivir/cyclodextrin complex on the international space station. Scientific Reports. https://doi.org/10.1038/s41598-024-81428-5
- Lee, H., Diao, J., Tian, Y., et al. (2025). Developing an alternative medium for in-space biomanufacturing. Nature Communications. https://doi.org/10.1038/s41467-025-56088-2
- Liu, X., Pataranutaporn, P., Fram, B., et al. (2025). Development and flight-testing of modular autonomous cultivation systems for biological plastics upcycling aboard the ISS. npj Microgravity. https://doi.org/10.1038/s41526-025-00463-2
- Plante, I., Daniels, V., Young, M., et al. (2025). The long-term stability of solid-state oral pharmaceuticals exposed to simulated intravehicular space radiation. npj Microgravity. https://doi.org/10.1038/s41526-025-00469-w
- Reindl, J., Abrantes, A. M., Ahire, V., et al. (2023). Molecular Radiation Biology. Radiobiology Textbook. https://doi.org/10.1007/978-3-031-18810-7_3
- Siew, K., Nestler, K. A., Nelson, C., et al. (2024). Cosmic kidney disease: an integrated pan-omic, physiological and morphological study into spaceflight-induced renal dysfunction. Nature Communications. https://doi.org/10.1038/s41467-024-49212-1
- Yates, K., Berliner, A. J., Makrygiorgos, G., et al. (2024). Nitrogen accountancy in space agriculture. npj Microgravity. https://doi.org/10.1038/s41526-024-00428-x