Organoid Intelligence (OI): Integrating Human Brain Organoids with Silicon Microchips to Create Novel Biocomputing Systems

The convergence of biotechnology and information technology has opened a frontier that was, until recently, the exclusive domain of science fiction: the creation of biocomputing systems. At the heart of this revolution is Organoid Intelligence (OI), an emerging field predicated on the integration of self-assembling 3D human brain organoids with high-density silicon microchips. These organoids, derived from human pluripotent stem cells, recapitulate key aspects of brain development, forming complex neural networks with diverse cell types. By culturing them on microelectrode arrays (MEAs) that can both record and stimulate neuronal activity, researchers are no longer just passive observers of neural development in a dish. They are creating sophisticated, bidirectional brain-computer interfaces (BCIs) that lay the groundwork for a new class of "wetware" computer. This article will explore the foundations of OI, proposing a speculative yet grounded framework where these systems are not merely models of the brain but are harnessed as unique computational devices, capable of learning and processing information in ways that are fundamentally different from traditional silicon-based AI.
The Building Blocks: Living Neural Networks and Silicon Interfaces
The feasibility of OI rests on two parallel technological achievements. The first is the maturation of human brain organoid technology. Advanced protocols now enable the generation of organoids containing a rich diversity of neuronal and glial cell types, including dopaminergic, GABAergic, glutamatergic, and serotonergic neurons, which are essential for complex network interactions (Smits et al., 2020). These neurons are not just a random assortment; they self-organize into structures that exhibit spontaneous, coordinated electrical activity, complex oscillatory dynamics, and functional connectivity reminiscent of developing neural circuits (Sharf et al., 2022).
The second key component is the development of sophisticated interfaces to read from and write to this biological hardware. High-density CMOS-based MEAs and nanopillar electrode arrays provide a non-invasive, high-resolution window into the organoid’s inner workings. These platforms can capture extracellular action potentials from thousands of neurons simultaneously, and with advancing technology, can even detect the subtle, sub-threshold electrical activities that precede neural firing (Shukla et al., 2024; Sharf et al., 2022). This high-fidelity I/O (input/output) is critical, transforming the organoid from a biological specimen into a functional component of a larger system—a processor whose internal state can be both monitored and manipulated.
From Neural Activity to "Wetware" Computation
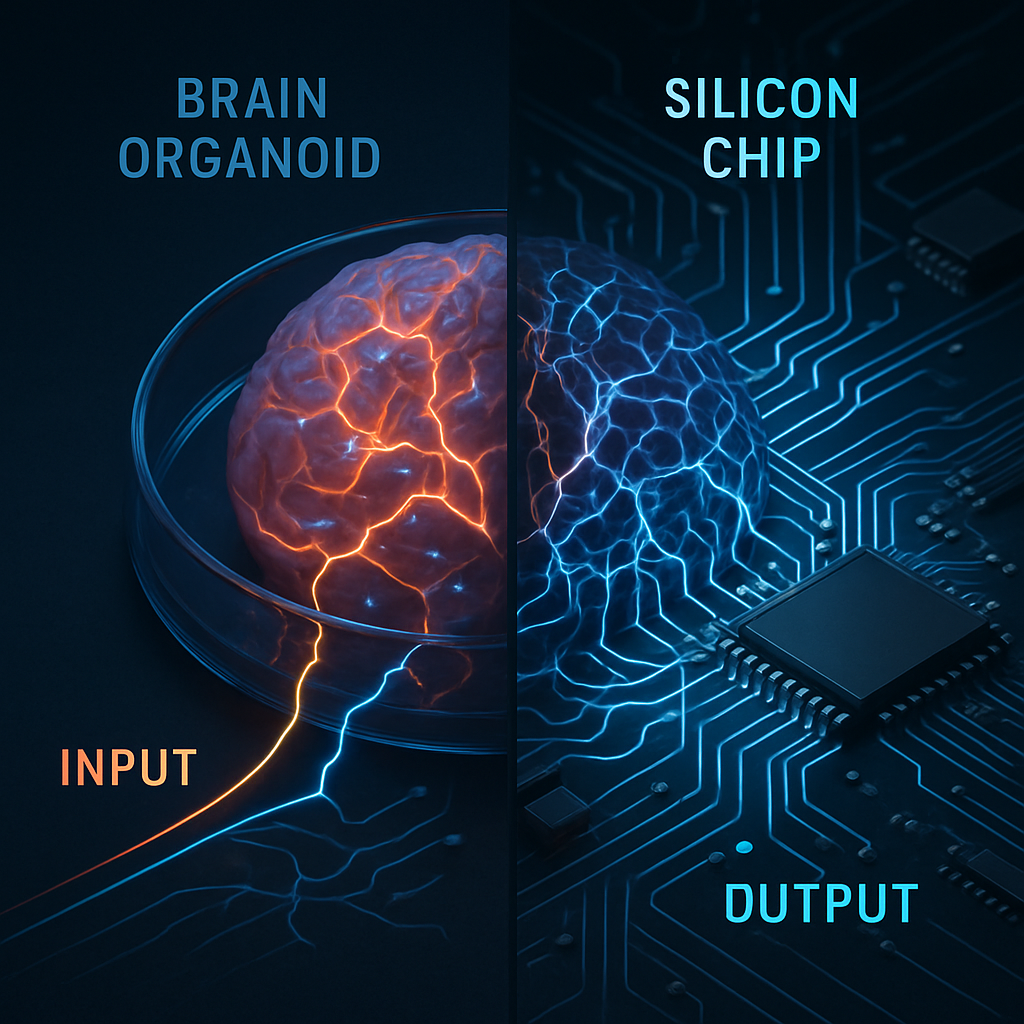
The mere presence of electrical activity does not equal computation. The conceptual leap of OI is to treat the brain organoid as a powerful, if inscrutable, information processor. A highly influential paradigm for understanding this is reservoir computing. In this model, a complex, high-dimensional dynamical system—the "reservoir"—is used to transform simple input signals into a much richer spatiotemporal pattern of activity. A simple "readout" layer can then be trained to interpret these complex patterns to perform tasks like classification or prediction.
We propose that a brain organoid on an MEA is the ultimate biological reservoir. Its intricate, recurrently connected network of diverse neurons provides a vast dynamical repertoire. The organoid’s inherent properties of neuroplasticity—the ability to strengthen or weaken connections in response to activity—provide a mechanism for learning and memory. A groundbreaking 2022 study demonstrated this principle by showing that a 2D culture of cortical neurons on an MEA could learn to play a simplified version of the video game Pong. The system, dubbed "DishBrain," was given sensory feedback about the ball's position via electrical stimulation. Through a process of self-organized learning based on minimizing prediction error, the culture altered its collective activity to control the paddle, demonstrating a primitive form of sentience and intelligence in a dish (Kagan et al., 2022). OI represents the next generation of this concept, moving from a 2D culture to a complex, 3D human brain organoid, vastly increasing the potential computational power and structural sophistication of the reservoir.
Speculative Framework: Training an Organoid Intelligence
Harnessing the organoid's computational potential requires a closed-loop system capable of goal-directed learning. We speculate that this can be achieved through a reinforcement learning framework. An OI system would receive input data encoded as precise patterns of electrical stimulation delivered to specific locations in the organoid via the MEA. This input would perturb the organoid's ongoing dynamics, creating a unique spatiotemporal "answer" in the form of neural activity, which is read by the MEA's recording electrodes.
A conventional machine learning algorithm would then serve as the "critic," comparing the organoid's output to a desired target outcome. If the output is "correct," no feedback is given. If it is "incorrect," the critic calculates an error signal, which is translated back into a new pattern of electrical stimulation—a "nudge"—delivered to the organoid. This feedback, repeated over many trials, would leverage the organoid's innate synaptic plasticity mechanisms (like spike-timing-dependent plasticity) to physically rewire its own circuitry, progressively tuning the network to become better at the task. In essence, we would not be programming the organoid, but rather providing the environmental conditions and feedback necessary for it to teach itself. Such a system could theoretically tackle problems that rely on complex pattern recognition or finding solutions in high-dimensional spaces, tasks where biological neural networks excel.
The Profound Frontier: Ethical and Philosophical Implications
The prospect of OI, particularly the term "intelligence," compels an immediate and serious consideration of the associated ethical and philosophical questions. While current organoids are nascent structures, lacking the scale, sensory inputs, and architecture of a conscious brain, the trajectory of this research is pointed toward systems of increasing complexity. Questions about potential sentience, suffering, or the moral status of a biological entity designed for computation are no longer theoretical. As we develop systems that can learn, remember, and adapt, we must proactively develop a robust ethical framework. This necessitates a multidisciplinary dialogue between neuroscientists, computer scientists, ethicists, and the public to establish clear guidelines and boundaries before we approach morally ambiguous territory. The goal of OI is not to create a "brain in a vat," but a new tool for science and technology; ensuring it remains a tool requires our utmost ethical vigilance.

Conclusion
Organoid Intelligence represents a paradigm shift, moving beyond using organoids as passive models of disease to actively integrating them into functional biocomputing systems. By merging the self-organizing, adaptive processing power of living human neural networks with the precision of silicon-based interfaces, OI offers a powerful new platform. In the near term, it could provide unprecedented insights into the mechanisms of learning, memory, and information processing in the human brain. It could also serve as a next-generation platform for testing therapeutics for neurological and psychiatric disorders by observing how drugs affect the network's computational function, not just its cellular health.
The long-term vision of OI as a new form of computer is ambitious and fraught with challenges, including vascularizing organoids for long-term stability, increasing the bandwidth of brain-computer communication, and navigating the profound ethical landscape. Yet, the foundational proofs of concept are in place. The fusion of the living and the digital is underway, promising to unlock computational principles that have been refined by billions of years of evolution and to redefine our understanding of intelligence itself.
References
- Chow, S. Y. A., Hu, H., Osaki, T., Levi, T., & Ikeuchi, Y. (2022). Advances in construction and modeling of functional neural circuits in vitro. Neurochemical Research. https://doi.org/10.1007/s11064-022-03682-1
- Kagan, B. J., Kitchen, A. C., Tran, N. T., Parker, B. J., Bhat, A., Rollo, B., Razi, A., & Friston, K. J. (2022). In vitro neurons learn and exhibit sentience when embodied in a simulated game-world. Neuron. https://doi.org/10.1016/j.neuron.2022.09.001
- Liu, Y., Xu, S., Yang, Y., Zhang, K., He, E., Liang, W., Luo, J., Wu, Y., & Cai, X. (2022). Nanomaterial-based microelectrode arrays for in vitro bidirectional brain–computer interfaces: a review. Microsystems & Nanoengineering. https://doi.org/10.1038/s41378-022-00479-8
- Sharf, T., van der Molen, T., Glasauer, S. M. K., Guzman, E., Buccino, A. P., Luna, G., Cheng, Z., Audouard, M., Ranasinghe, K. G., Kudo, K., Nagarajan, S. S., Tovar, K. R., Petzold, L. R., Hierlemann, A., Hansma, P. K., & Kosik, K. S. (2022). Functional neuronal circuitry and oscillatory dynamics in human brain organoids. Nature Communications. https://doi.org/10.1038/s41467-022-32115-4
- Shukla, S., Schwartz, J. L., Walsh, C., Wong, W. M., Patel, V., Hsieh, Y.-P., Onwuasoanya, C., Chen, S., Offenhäusser, A., Cauwenberghs, G., Santoro, F., Muotri, A. R., Yeo, G. W., Chalasani, S. H., & Jahed, Z. (2024). Supra- and sub-threshold intracellular-like recording of 2D and 3D neuronal networks using nanopillar electrode arrays. Microsystems & Nanoengineering. https://doi.org/10.1038/s41378-024-00817-y
- Smits, L. M., Magni, S., Kinugawa, K., Grzyb, K., Luginbühl, J., Sabate-Soler, S., Bolognin, S., Shin, J. W., Mori, E., Skupin, A., & Schwamborn, J. C. (2020). Single-cell transcriptomics reveals multiple neuronal cell types in human midbrain-specific organoids. Cell and Tissue Research. https://doi.org/10.1007/s00441-020-03249-y
- Suárez, L. E., Mihalik, A., Milisav, F., Marshall, K., Li, M., Vértes, P. E., Lajoie, G., & Misic, B. (2024). Connectome-based reservoir computing with the conn2res toolbox. Nature Communications. https://doi.org/10.1038/s41467-024-44900-4