Mycoremediation of Persistent Organic Pollutants

Persistent Organic Pollutants (POPs) encompass a diverse group of hazardous chemical substances, including pesticides (e.g., DDT, lindane), industrial chemicals (e.g., PCBs, dioxins), and unintended byproducts (e.g., polycyclic aromatic hydrocarbons - PAHs). Their defining characteristics—persistence in the environment, bioaccumulation in food chains, toxicity to humans and wildlife, and capacity for long-range environmental transport—make them a significant global concern. Conventional remediation techniques for POP-contaminated sites are often costly, energy-intensive, and can lead to secondary pollution. Mycoremediation, the use of fungi and their metabolic processes to degrade or sequester contaminants, has emerged as a highly promising, eco-friendly, and cost-effective alternative. Fungi, particularly white-rot fungi, possess robust enzymatic systems capable of breaking down the complex and recalcitrant structures of many POPs.
This article delves into the current understanding and cutting-edge advancements in the mycoremediation of POPs. It explores the diverse enzymatic and non-enzymatic mechanisms employed by fungi, highlights key fungal genera demonstrating significant degradative potential, discusses innovative strategies to enhance remediation efficacy, and critically examines the existing challenges and underexplored opportunities. By synthesizing current knowledge and proposing novel hypotheses, this review aims to illuminate the path towards harnessing fungal capabilities for effectively tackling the pervasive issue of POP pollution.
Fungal Arsenal: Mechanisms of POP Degradation
Fungi employ a multifaceted strategy to attack and neutralize POPs, primarily driven by their powerful extracellular ligninolytic enzyme systems. These enzymes, evolved to degrade the complex polymer lignin in wood, are non-specific and can fortuitously degrade a wide array of structurally similar pollutants, including POPs. The key players are laccases (Lac), manganese peroxidases (MnP), and lignin peroxidases (LiP). Laccases are multi-copper oxidases that catalyze the oxidation of phenolic compounds and anilines, often using mediators to expand their substrate range to non-phenolic POPs. MnP utilizes Mn(II) as a substrate, oxidizing it to Mn(III), which then acts as a diffusible oxidizer of phenolic and non-phenolic compounds. LiP, with its high redox potential, can directly oxidize non-phenolic aromatic rings, initiating the degradation cascade (Agrawal et al., 2018; Pundir et al., 2024; Egbewale et al., 2024).
Beyond extracellular enzymes, the intracellular cytochrome P450 monooxygenase (CYP450) system plays a crucial role, particularly in the initial transformation of POPs. These enzymes introduce oxygen atoms into the pollutant molecule, increasing its polarity and susceptibility to further degradation by other enzymes (Dey et al., 2024). Fungal biomass itself also contributes through biosorption and bioaccumulation, where POPs are adsorbed onto the fungal cell wall or taken up into the cell, effectively sequestering them from the environment (Upadhyay et al., 2024). Furthermore, fungi can degrade POPs via co-metabolism, where the pollutant is broken down incidentally during the metabolism of a primary growth substrate, a common scenario when fungi are grown on lignocellulosic materials (Hultberg & Golovko, 2024; Omoni et al., 2024).
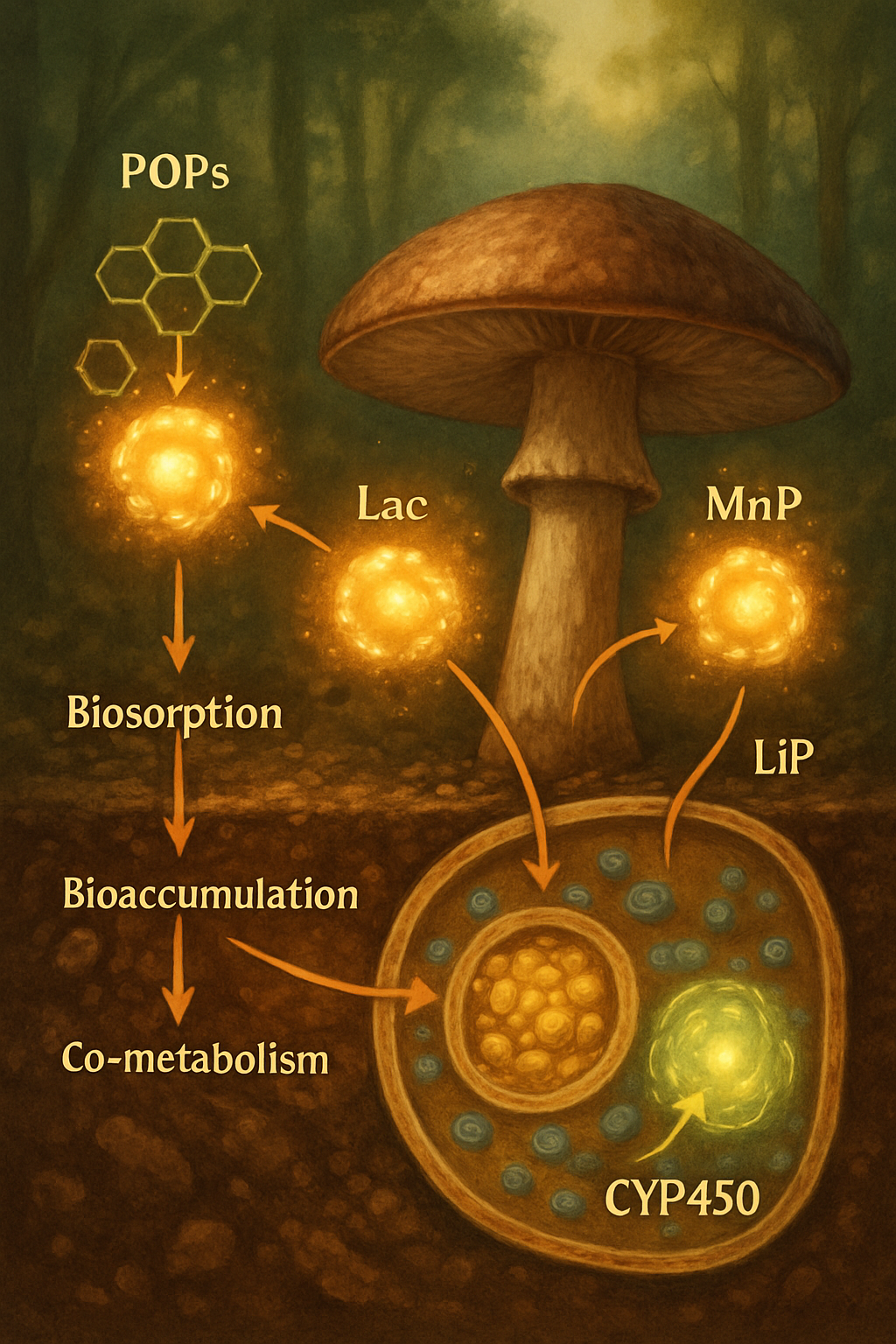
Key Fungal Players in POP Mycoremediation
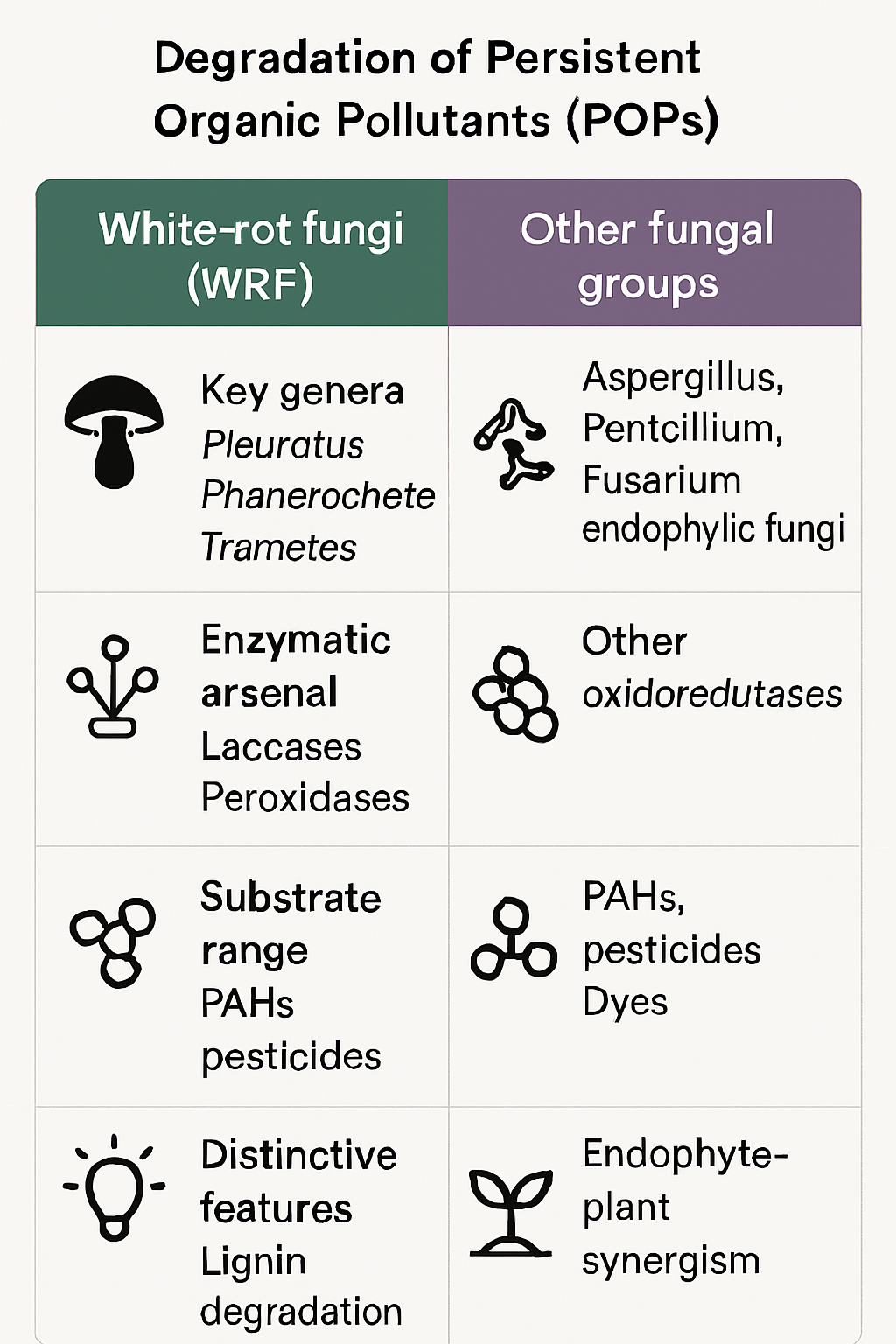
White-rot fungi (WRF), belonging to the Basidiomycota, are the most extensively studied and effective group for POP mycoremediation due to their robust ligninolytic enzyme machinery. Genera such as Pleurotus (oyster mushrooms), Trametes (turkey tail), Phanerochaete, and Ganoderma have demonstrated significant capabilities in degrading a wide spectrum of POPs, including PAHs, pesticides, polychlorinated biphenyls (PCBs), and synthetic dyes which often share structural similarities with POPs (Agrawal et al., 2018; Ibrahim et al., 2024; Hultberg & Golovko, 2024). For instance, Ganoderma lucidum has been shown to efficiently degrade phenanthrene and pyrene, producing significant amounts of laccase, LiP, and MnP (Agrawal et al., 2018).
While WRF are champions, other fungal groups also contribute significantly. Species from genera like Aspergillus, Penicillium, and Fusarium (Ascomycota and Deuteromycota) possess diverse metabolic pathways and have been found effective against specific POPs, including certain pesticides and industrial dyes (Ghanaim et al., 2024; Dey et al., 2024; Magnoli et al., 2023; Serag et al., 2025). Aspergillus flavus, for example, efficiently degrades various azo dyes through enzymatic action (Ghanaim et al., 2024). Endophytic fungi, which live symbiotically within plant tissues, represent an emerging frontier. Their intimate association with plants could facilitate in-planta degradation of POPs or enhance phytoremediation processes by degrading pollutants taken up by the host plant, potentially offering a synergistic approach to soil and water cleanup (Bhardwaj, 2025; Obi et al., 2024). This highlights a novel integrative strategy where plant-fungal partnerships could be engineered for more effective pollutant removal.
Innovations and Strategies for Enhanced Mycoremediation
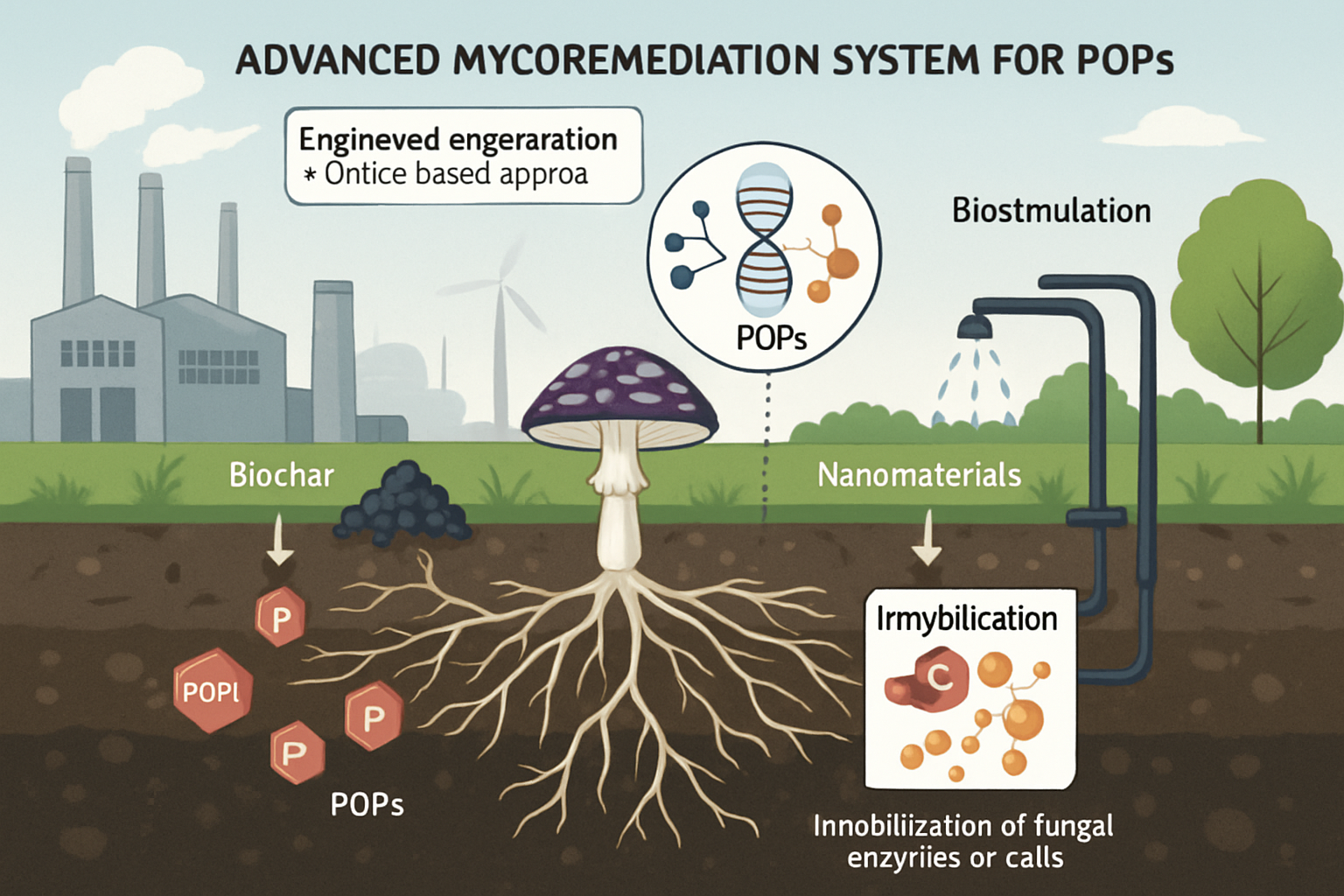
Maximizing the efficiency of mycoremediation requires innovative approaches. Bioaugmentation, the introduction of specific, highly efficient fungal strains or consortia to a contaminated site, and biostimulation, the addition of nutrients or growth substrates (e.g., lignocellulosic waste) to stimulate native or introduced fungal activity, are foundational strategies (Matilda & Samuel, 2024; Omoni et al., 2024). Immobilization techniques, where fungal cells or their enzymes are encapsulated or attached to a support matrix (e.g., hydrogels, biochar), can enhance enzyme stability, facilitate biomass recovery, and improve operational control in bioreactor systems (Upadhyay et al., 2024; Zhang et al., 2025). Such immobilized systems could be particularly effective for continuous treatment of industrial effluents containing POPs.
The advent of genetic engineering and 'omics' technologies (genomics, transcriptomics, proteomics, metabolomics) offers powerful tools to tailor fungi for superior POP degradation. These approaches can be used to identify novel degradative enzymes, understand regulatory networks, and engineer strains for enhanced enzyme production, broader substrate specificity, or increased tolerance to toxic pollutants and harsh environmental conditions (Dey et al., 2024; Kumar et al., 2025; Wong et al., 2025). For instance, proteomics has been used to unveil cellular responses and key enzymes in Aspergillus fumigatus during lindane degradation (Dey et al., 2024). Furthermore, developing fungal consortia, potentially with bacteria or microalgae, can lead to synergistic degradation of complex POP mixtures, as different organisms may tackle different steps in the degradation pathway or detoxify inhibitory intermediates (Rathour et al., 2024). The integration of mycoremediation with other technologies, such as biochar amendment (which can sorb POPs and act as a fungal habitat) or nanomaterials (as catalyst supports or enzyme carriers), also shows promise for enhancing overall remediation outcomes (Zhang et al., 2023; Dabas, 2025).
Bridging Gaps: Challenges, Novel Hypotheses, and Untapped Potential
Despite significant progress, several challenges hinder the widespread application of mycoremediation for POPs. The bioavailability of POPs in soil and sediment is a major limitation, as these hydrophobic compounds tend to sorb strongly to organic matter and clay particles, making them less accessible to fungal enzymes. The toxicity of high concentrations of POPs or their degradation intermediates can inhibit fungal growth and enzymatic activity. Competition with native soil microflora and the complexities of scaling up lab-scale successes to effective and predictable field-scale applications, including inoculum production, delivery, and long-term survival and activity, remain significant hurdles (Pundir et al., 2024; Magnoli et al., 2023). A critical, often overlooked aspect is the potential for incomplete degradation, leading to the formation of new metabolites that may be as toxic, or even more toxic, than the parent POP. Comprehensive toxicological assessment of treated matrices is therefore essential (Upadhyay et al., 2024).
A largely underexplored area is the potential of extremophilic fungi. Fungi isolated from extreme environments (e.g., high salinity, extreme pH, high temperatures, or pollutant-rich sites) possess unique enzymatic and physiological adaptations that could make them inherently more robust and efficient for degrading recalcitrant POPs in challenging industrial waste streams or co-contaminated sites (Farouk et al., 2025; Chia et al., 2024). It is hypothesized that extremophilic fungi, with their specialized enzymes and stress-response mechanisms, could offer superior degradation rates and resilience when dealing with highly persistent POPs or complex pollutant mixtures that inhibit conventional mesophilic fungi. Another significant untapped potential lies in the mycoremediation of 'forever chemicals' like per- and polyfluoroalkyl substances (PFAS). While current research is limited, the broad specificity and oxidative power of fungal ligninolytic enzymes, particularly laccases and peroxidases, suggest they could be candidates for breaking the highly stable C-F bonds in PFAS, a critical research need identified in recent reviews (Pundir et al., 2024). This points to a nascent but potentially high-impact research direction.

Conclusion
Mycoremediation presents a compelling, environmentally sound, and potentially low-cost strategy for addressing the global challenge of POP contamination. The diverse enzymatic machinery of fungi, especially white-rot fungi, offers a natural solution for transforming these persistent and toxic chemicals into less harmful substances. Key implications include the detoxification of contaminated soils, sediments, and industrial effluents, contributing to environmental restoration and human health protection. However, realizing the full potential of mycoremediation requires concerted research efforts.
Future directions should focus on: (1) Discovering and characterizing novel fungal strains and enzymes with superior POP-degrading capabilities, particularly from unique or extreme environments. (2) Leveraging genetic and metabolic engineering, guided by 'omics' data, to develop hyper-efficient fungal biocatalysts. (3) Designing and optimizing robust bioreactor systems and in-situ application strategies for diverse environmental matrices. (4) Deepening our understanding of fungal ecology, pollutant bioavailability, and degradation pathways in complex, real-world contaminated environments. (5) Rigorous and standardized assessment of metabolite formation and toxicity to ensure complete detoxification. An open problem remains the effective degradation of complex mixtures of POPs, often found in contaminated sites, which may require multi-species consortia or integrated treatment trains. A provocative question for the future is: Can we engineer synergistic fungal-based systems that not only remediate POPs but also valorize the breakdown products or the fungal biomass itself into valuable bioproducts (e.g., biofuels, platform chemicals, or bio-based materials like mycelium composites), thereby transforming a pollution problem into a resource opportunity and fostering a circular bioeconomy approach to waste management? (Wattanavichean et al., 2025; Omoni et al., 2024; Nascimento Deschamps et al., 2024). Successful translation of mycoremediation technologies from the lab to the field will ultimately depend on interdisciplinary collaboration, addressing scalability, and ensuring regulatory and public acceptance.
References
- Agrawal, Nikki et al. (2018). Degradation of polycyclic aromatic hydrocarbons (phenanthrene and pyrene) by the ligninolytic fungi Ganoderma lucidum isolated from the hardwood stump. Bioresources and Bioprocessing.
- Bhardwaj, Ankur (2025). Endophytes unleashed: natural allies for heavy metal bioremediation. Discover Plants.
- Chia, Xing Kai et al. (2024). Role of Extremophiles in Biodegradation of Emerging Pollutants. Topics in Catalysis.
- Cucu, Maria Alexandra et al. (2024). Microbial diversity and cover plants in de-sealed urban soil as strategies for mitigating anthropogenic volatile organic compounds. Discover Soil.
- Dabas, Seema (2025). Metal and metal oxide based nanoremediation: a sustainable alternative. Discover Materials.
- Dey, Priyadarshini et al. (2024). Unveiling fungal strategies: Mycoremediation in multi-metal pesticide environment using proteomics. Scientific Reports.
- Egbewale, Samson O. et al. (2024). Anthracene detoxification by Laccases from indigenous fungal strains Trichoderma lixii FLU1 and Talaromyces pinophilus FLU12. Biodegradation.
- Elshafei, Ali Mohamed & Mansour, Rawia (2024). Microbial bioremediation of soils contaminated with petroleum hydrocarbons. Discover Soil.
- Farouk, Noura I. et al. (2025). Adaptation strategies in haloalkaliphilic fungi: Aspergillus salinarum, cladosporium sphaerospermum, and penicillium camemberti. BMC Microbiology.
- Ghanaim, Amira M. et al. (2024). Biodegradation of azo dyes by Aspergillus flavus and its bioremediation potential using seed germination efficiency. BMC Microbiology.
- Haller, Henrik et al. (2021). Polluted lignocellulose-bearing sediments as a resource for marketable goods—a review of potential technologies for biochemical and thermochemical processing and remediation. Clean Technologies and Environmental Policy.
- Hultberg, M. & Golovko, O. (2024). Use of sawdust for production of ligninolytic enzymes by white-rot fungi and pharmaceutical removal. Bioprocess and Biosystems Engineering.
- Ibrahim, Ahmed E. et al. (2024). Environmental implications of three Pleurotus strain growths for water remediation in the perspective of climate change in New Egyptian Delta. Environmental Science and Pollution Research.
- Košnář, Zdeněk & Tlustoš, Pavel (2025). Fly bioash-borne polycyclic aromatic hydrocarbon removal by rapid-growth remediation systems of poplar, industrial hemp and parsley. Environmental Sciences Europe.
- Kumar, Mukesh et al. (2025). Advanced biotechnological tools towards achieving United Nations Sustainable Development Goals (UNSDGs) for mitigation of microplastics from environments: a review. Discover Sustainability.
- Magnoli, Karen et al. (2023). Fungal biodegradation of chlorinated herbicides: an overview with an emphasis on 2,4-D in Argentina. Biodegradation.
- Matilda, Manasseh Ilumunter & Samuel, Humphrey Sam (2024). Bioremediation of oil spill: concept, methods and applications. Discover Chemistry.
- Mohamadpour, Fahimeh & Mohamadpour, Farzaneh (2024). Photodegradation of six selected antipsychiatric drugs; carbamazepine, sertraline, amisulpride, amitriptyline, diazepam, and alprazolam in environment: efficiency, pathway, and mechanism—a review. Sustainable Environment Research.
- Nascimento Deschamps, Joara Lúcia et al. (2024). Sustainable production of Pleurotus sajor-caju mushrooms and biocomposites using brewer’s spent and agro-industrial residues. Scientific Reports.
- Obi, Linda U. et al. (2024). Application of Endophytes in Bioremediation, Biotransformation, and Water Disinfection for Irrigation Systems. Enhancing Water and Food Security Through Improved Agricultural Water Productivity.
- Omoni, Victor Taghoghor et al. (2024). Enhanced Remediation of Polycyclic Aromatic Hydrocarbons in Soil Through Fungal Delignification Strategy and Organic Waste Amendment: A Review. Indian Journal of Microbiology.
- Pundir, Ashok et al. (2024). Fungi as versatile biocatalytic tool for treatment of textile wastewater effluents. Environmental Sciences Europe.
- Rathour, Ranju Kumari et al. (2024). Bacterial–microalgal consortia for bioremediation of textile industry wastewater and resource recovery for circular economy. Biotechnology for the Environment.
- Serag, Mamdouh S. et al. (2025). Optimizing Wastewater Treatment Using Endophytic Fungi Isolated from Acacia saligna L. Bark. Beni-Suef University Journal of Basic and Applied Sciences.
- Upadhyay, R. et al. (2024). Myco-remediation of synthetic dyes: a comprehensive review on contaminant alleviation mechanism, kinetic study and toxicity analysis. International Journal of Environmental Science and Technology.
- Vunduk, Jovana et al. (2025). The application of laccase-rich extract of spent mushroom substrates for removing lignin from jute fabric waste: a dual management approach. Scientific Reports.
- Wattanavichean, Nungnit et al. (2025). Mycelium-Based Breakthroughs: Exploring Commercialization, Research, and Next-Gen Possibilities. Circular Economy and Sustainability.
- Wong, Ryan Wei Kwan et al. (2025). Mining yeast diversity unveils novel targets for improved heterologous laccase production in Saccharomyces cerevisiae. Microbial Cell Factories.
- Zhang, Jinlong et al. (2025). Advanced enzyme-assembled hydrogels for the remediation of contaminated water. Nature Communications.
- Zhang, Meng et al. (2023). Insights into the mechanisms underlying the biodegradation of phenanthrene in biochar-amended soil: from bioavailability to soil microbial communities. Biochar.