Mechanobiology of Cellular Extrusion: Force-Driven Epithelial Homeostasis
Epithelial tissues form essential barriers and functional surfaces in multicellular organisms. Maintaining their integrity and functionality in the face of cell damage, overcrowding, or the emergence of aberrant cells is critical. Cellular extrusion is a fundamental biological process by which unwanted or superfluous cells are actively removed from an epithelial layer without compromising its barrier function. This process is pivotal for tissue homeostasis, embryonic development, and defense against diseases like cancer by eliminating transformed cells. While biochemical signals undoubtedly play a role, a growing body of evidence highlights that cellular extrusion is profoundly governed by mechanical forces and the intricate interplay of mechanotransduction pathways. This article explores the mechanobiology of cellular extrusion, focusing on how forces are generated, sensed, and orchestrated to drive epithelial homeostasis.
The Machinery of Extrusion: Intrinsic and Extrinsic Force Generation
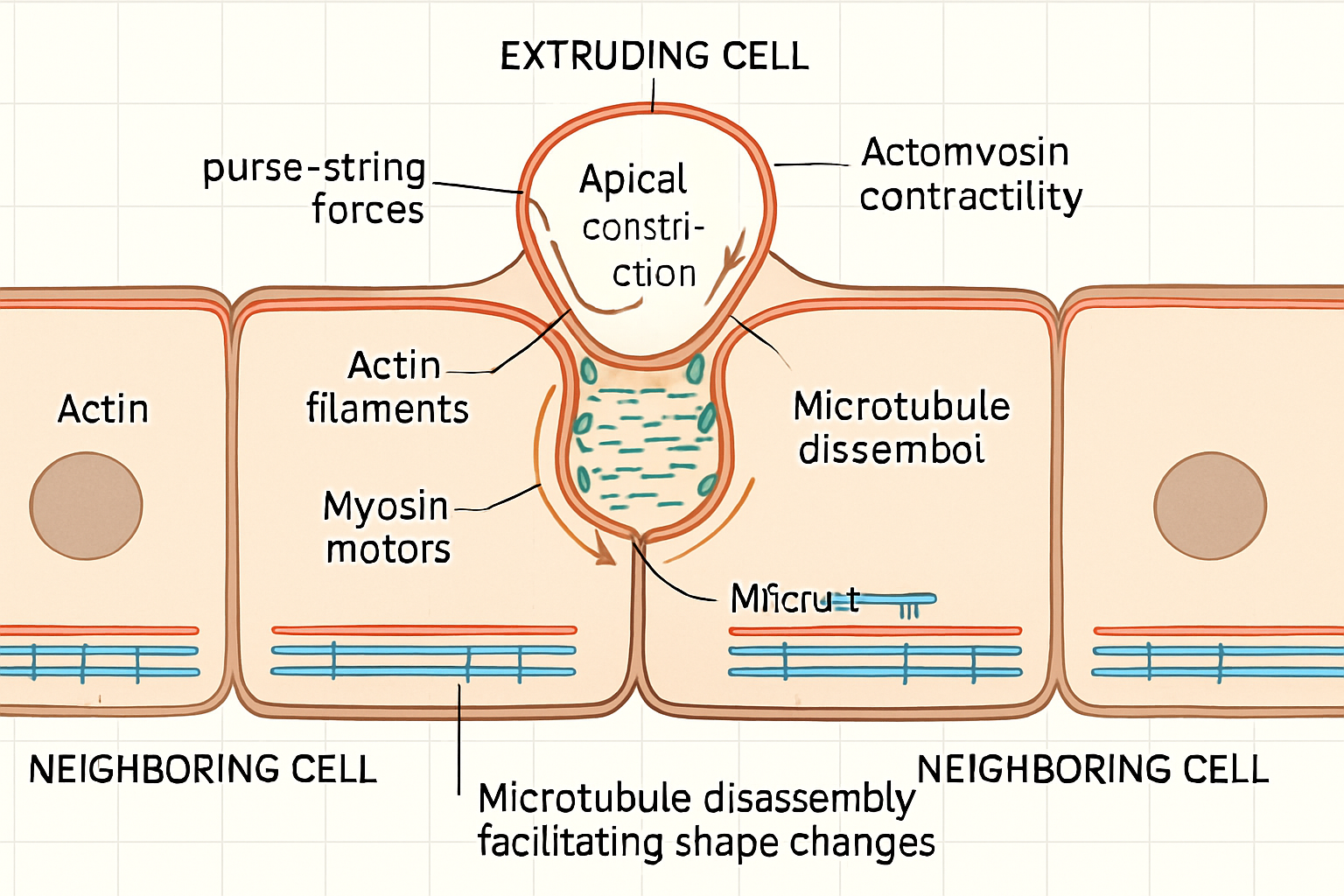
The removal of a cell from an epithelium is an active mechanical event requiring coordinated force generation. A primary driver is the actomyosin cytoskeleton. Apical actomyosin contraction in the extruding cell and its neighbors generates a purse-string-like force that constricts the apical surface of the target cell, facilitating its ejection, typically apically. Studies have shown that non-muscle myosin II is crucial for this process, with its activity being precisely regulated at specific cell-cell junctions. For instance, Prakash et al. (2025) demonstrated how differential junctional contractility, modulated by myosin II, underpins the organization and planar polarity in the auditory epithelium, suggesting a similar role in controlled cell removal. Furthermore, Schoenit et al. (2025) revealed that increased mechanical activity and stress fluctuations at the interface between competing cell types can lead to upward forces and cell elimination, underscoring the direct role of force in extrusion. Beyond actomyosin contractility, other cytoskeletal components are involved. Villars et al. (2022) discovered that microtubule disassembly by caspases is an important rate-limiting step in apoptotic cell extrusion in Drosophila. This suggests that the microtubule network, typically associated with cell shape and stability, must be actively dismantled to permit the cell shape changes necessary for extrusion. This finding opens questions about the role of microtubules in live-cell extrusion, where apoptotic pathways may not be the primary trigger. The interplay between actin-based protrusive forces and microtubule dynamics in coordinating the complex cell shape changes during extrusion remains an active area of research. Moreover, the mechanical properties of the surrounding tissue, including the viscoelasticity of the extracellular matrix (Courbot & Elosegui-Artola, 2025), likely influence the forces required for, and the efficiency of, cellular extrusion.
Sensing and Responding to Mechanical Cues: Mechanotransduction in Extrusion
Cells within an epithelium are not passive entities; they actively sense their mechanical environment. Overcrowding, for example, leads to increased compressive stress and changes in cell shape, which can trigger extrusion of excess cells to maintain optimal cell density. Mechanosensitive ion channels, such as Piezo1, are implicated in sensing these mechanical stresses. When activated by membrane stretch or force, these channels can initiate signaling cascades that lead to extrusion. For instance, Moshiri et al. (2023) found that the extrusion of Enterovirus A71-infected cells from colonic organoids is dependent on force sensing via mechanosensitive ion channels, rather than solely apoptosis. This suggests a direct mechanotransductive pathway where viral infection might alter cellular mechanics or induce forces that are then sensed to trigger extrusion as a defense mechanism.
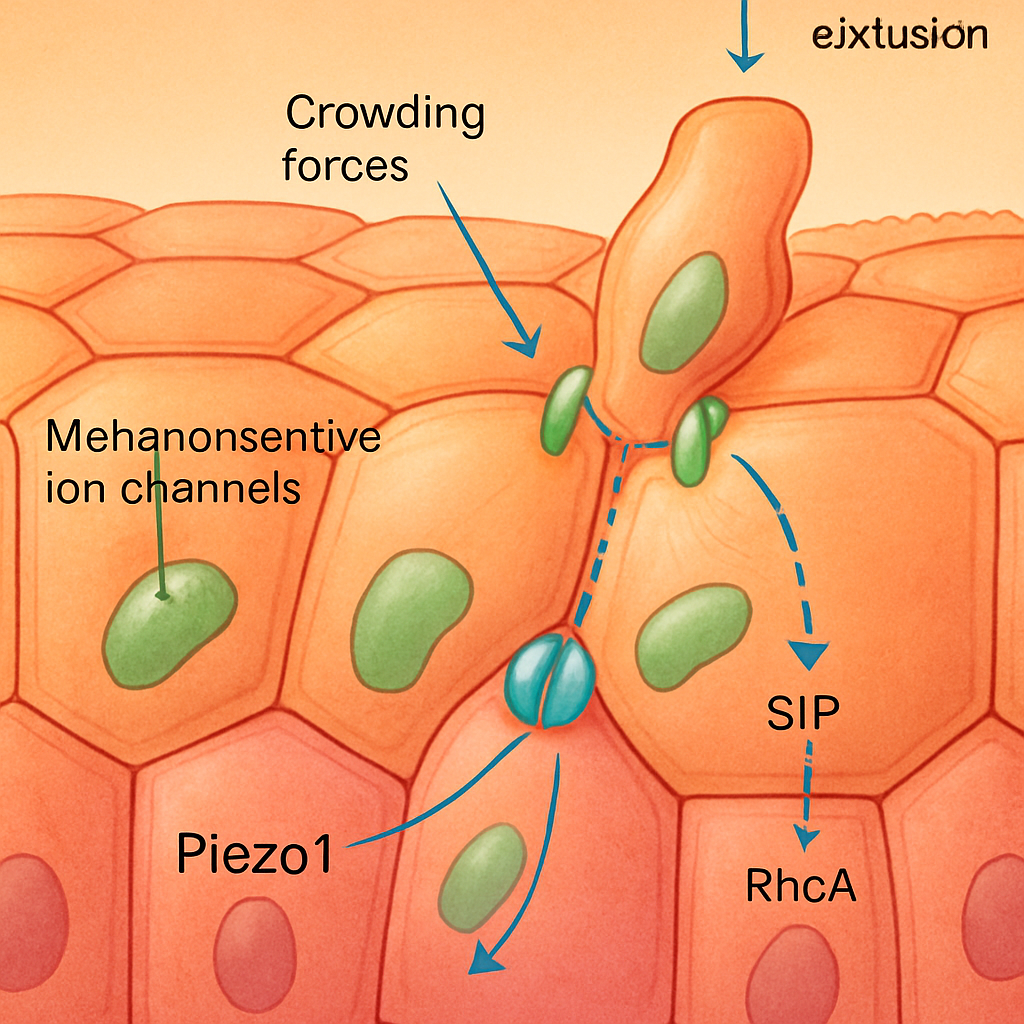
The Sphingosine-1-phosphate (S1P) signaling pathway is another critical mediator of mechanically induced extrusion, particularly in response to crowding. S1P gradients, established by the differential expression of S1P-producing kinases and S1P-degrading lyases, guide the extrusion process. Cells sense these gradients, leading to the activation of RhoA and subsequent actomyosin contraction in neighboring cells, which helps push out the target cell. The Hippo pathway, a key regulator of tissue growth and cell fate (Nita & Moroishi, 2024), also interfaces with mechanotransduction and has been implicated in processes related to cell competition and homeostasis, potentially playing a role in deciding which cells are to be extruded based on mechanical inputs and cell fitness. The coordination of nucleus size and epithelial cell morphology heterogeneity has also been shown to impact chromatin state diversity (Bermudez et al., 2025), suggesting that underlying mechanical constraints can have profound effects on cellular identity and, potentially, their susceptibility to extrusion.
Mechanical Cell Competition and Tissue-Level Force Dynamics
Cellular extrusion is not always a solitary event initiated by a single compromised cell. It often occurs in the context of "mechanical cell competition," where cells with different mechanical properties or fitness levels compete for space and survival within the epithelium. Schoenit et al. (2025) elegantly demonstrated that differences in force transmission capabilities, often linked to the strength of intercellular adhesions (e.g., cadherins), are master regulators of mechanical cell competition. Cells with stronger adhesions and more efficient force transmission can outcompete and extrude their "less fit" neighbors. This process is vital for eliminating damaged, oncogenically transformed, or otherwise aberrant cells, thereby maintaining tissue health.
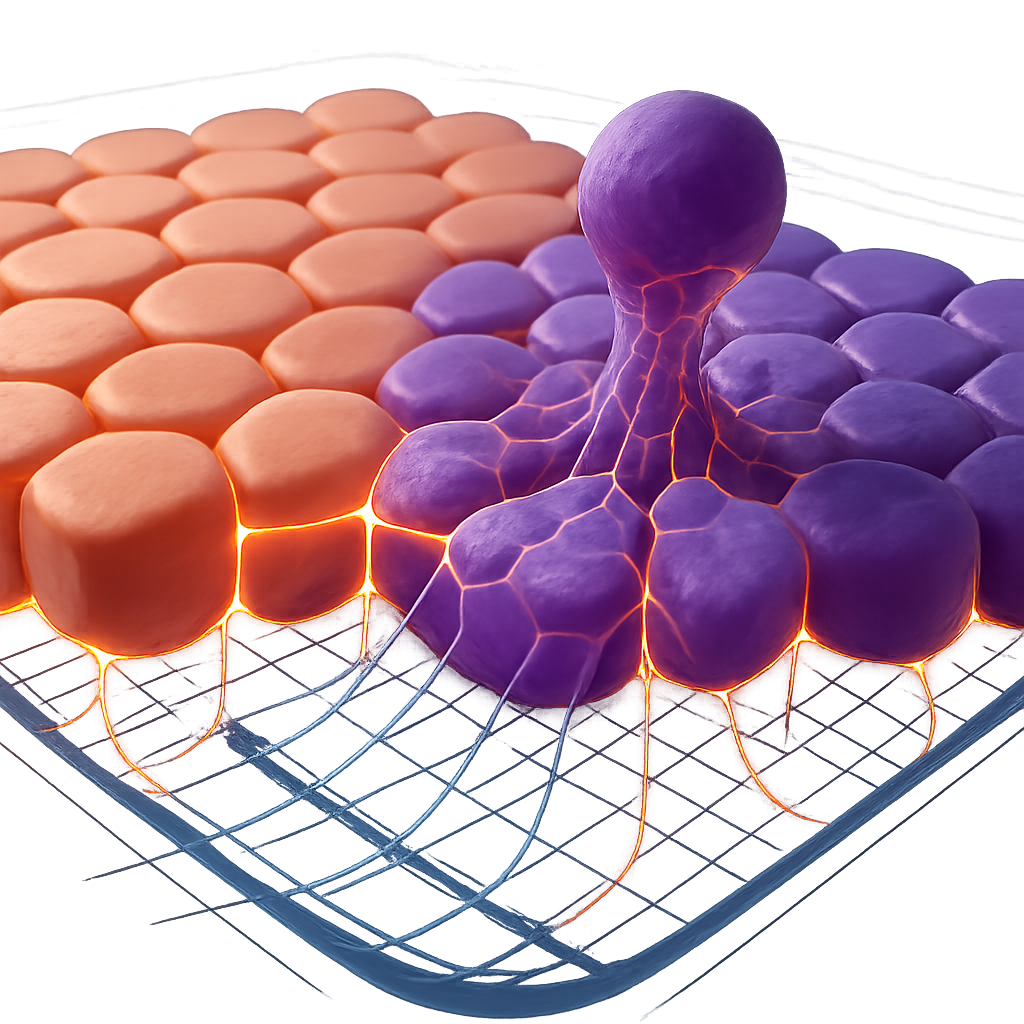
The forces involved in extrusion are not confined to the extruding cell and its immediate neighbors. Force transmission can occur across considerable distances within an epithelial sheet. The collective behavior of cells, influenced by active motility and intercellular mechanical coupling, dictates the overall stress distribution within the tissue. Gauquelin et al. (2024) showed mechanical convergence in mixed populations of epithelial cells, where traction and intercellular stresses became nearly indistinguishable between different cell types when mixed, highlighting the importance of the collective mechanical environment. Heuberger et al. (2025) showed that IFN-γ-driven extrusion of surface colonocytes leads to stromal remodeling, indicating a direct link between extrusion events and broader tissue-level mechanical and structural adaptations. Understanding these tissue-level force dynamics is crucial, as they can dictate the threshold for extrusion, the directionality (apical vs. basal), and the efficiency of the process.
Conclusion
The mechanobiology of cellular extrusion is a rapidly evolving field revealing the critical role of physical forces in maintaining epithelial homeostasis. Actomyosin contractility, microtubule dynamics, mechanosensitive signaling pathways like S1P and Piezo1, and mechanical cell competition are key elements in this force-driven process. The ability of epithelia to sense and respond to mechanical cues—be it crowding, damage, or the presence of unfit cells—and to precisely regulate force generation and transmission is paramount for development, tissue repair, and defense against disease. Future research will likely focus on a more integrated understanding of how different force-generating mechanisms are coordinated and how various mechanotransduction pathways are integrated to ensure robust and context-specific extrusion. For example, are there distinct mechanical signatures or thresholds that differentiate apoptotic extrusion from live-cell extrusion or viral-induced extrusion? The work by Villars et al. (2022) on microtubule disassembly in apoptotic extrusion begs the question of its role in other extrusion contexts. A compelling hypothesis is that the specific nature of the "extrusion signal" (e.g., apoptotic caspase activation vs. extreme crowding vs. aberrant oncogene expression) may recruit distinct combinations of force-generating modules and signaling pathways. The development of advanced imaging techniques to visualize forces in real-time within living tissues, coupled with sophisticated computational modeling (Alsubaie & Neufeld, 2024), will be crucial for dissecting these complex dynamics. Moreover, understanding the mechanical dialogue between the extruding cell, its neighbors, and the underlying extracellular matrix could reveal new checkpoints and regulatory mechanisms. An open problem is to fully elucidate how the decision between apical versus basal extrusion is mechanically determined and what the distinct physiological consequences of each route are, particularly in the context of cancer progression where basal extrusion can contribute to invasion. Ultimately, a deeper understanding of the mechanobiology of cellular extrusion holds promise for developing novel therapeutic strategies that, for instance, could selectively promote the extrusion of early-stage cancer cells or modulate extrusion to enhance tissue regeneration.
References
- Alsubaie, F. S., & Neufeld, Z. (2024). Modelling the effect of cell motility on mixing and invasion in epithelial monolayers. Journal of Biological Physics. https://doi.org/10.1007/s10867-024-09660-8
- Amiri, S. et al. (2023). Intracellular tension sensor reveals mechanical anisotropy of the actin cytoskeleton. Nature Communications. https://doi.org/10.1038/s41467-023-43612-5
- Bermudez, A. et al. (2025). Regulation of chromatin modifications through coordination of nucleus size and epithelial cell morphology heterogeneity. Communications Biology. https://doi.org/10.1038/s42003-025-07677-w
- Courbot, O., & Elosegui-Artola, A. (2025). The role of extracellular matrix viscoelasticity in development and disease. npj Biological Physics and Mechanics. https://doi.org/10.1038/s44341-025-00014-6
- Gauquelin, E. et al. (2024). Mechanical convergence in mixed populations of mammalian epithelial cells. The European Physical Journal E. https://doi.org/10.1140/epje/s10189-024-00415-w
- Heuberger, J. et al. (2025). Extrusion of BMP2+ surface colonocytes promotes stromal remodeling and tissue regeneration. Nature Communications. https://doi.org/10.1038/s41467-025-59474-y
- Moshiri, J. et al. (2023). Mechanosensitive extrusion of Enterovirus A71-infected cells from colonic organoids. Nature Microbiology. https://doi.org/10.1038/s41564-023-01339-5
- Nita, A., & Moroishi, T. (2024). Hippo pathway in cell–cell communication: emerging roles in development and regeneration. Inflammation and Regeneration. https://doi.org/10.1186/s41232-024-00331-8
- Prakash, A. et al. (2025). Junctional force patterning drives both positional order and planar polarity in the auditory epithelia. Nature Communications. https://doi.org/10.1038/s41467-025-58557-0
- Schoenit, A. et al. (2025). Force transmission is a master regulator of mechanical cell competition. Nature Materials. https://doi.org/10.1038/s41563-025-02150-9
- Ventura, G. et al. (2022). Multiciliated cells use filopodia to probe tissue mechanics during epithelial integration in vivo. Nature Communications. https://doi.org/10.1038/s41467-022-34165-0
- Villars, A. et al. (2022). Microtubule disassembly by caspases is an important rate-limiting step of cell extrusion. Nature Communications. https://doi.org/10.1038/s41467-022-31266-8
- Zhang, M., & Zhang, B. (2025). Extracellular matrix stiffness: mechanisms in tumor progression and therapeutic potential in cancer. Experimental Hematology & Oncology. https://doi.org/10.1186/s40164-025-00647-2
- Zhu, Y. et al. (2025). Epithelial cell competition is promoted by signaling from immune cells. Nature Communications. https://doi.org/10.1038/s41467-025-59130-5
- Chaithanya, KVS. et al. (2025). Cell-Level Modelling of Homeostasis in Confined Epithelial Monolayers. Journal of Elasticity. https://doi.org/10.1007/s10659-025-10120-0