Magneto-Acoustic Catalysis for the Degradation of Per- and Polyfluoroalkyl Substances (PFAS): A Sonochemical Approach to Breaking C-F Bonds in Contaminated Waterways

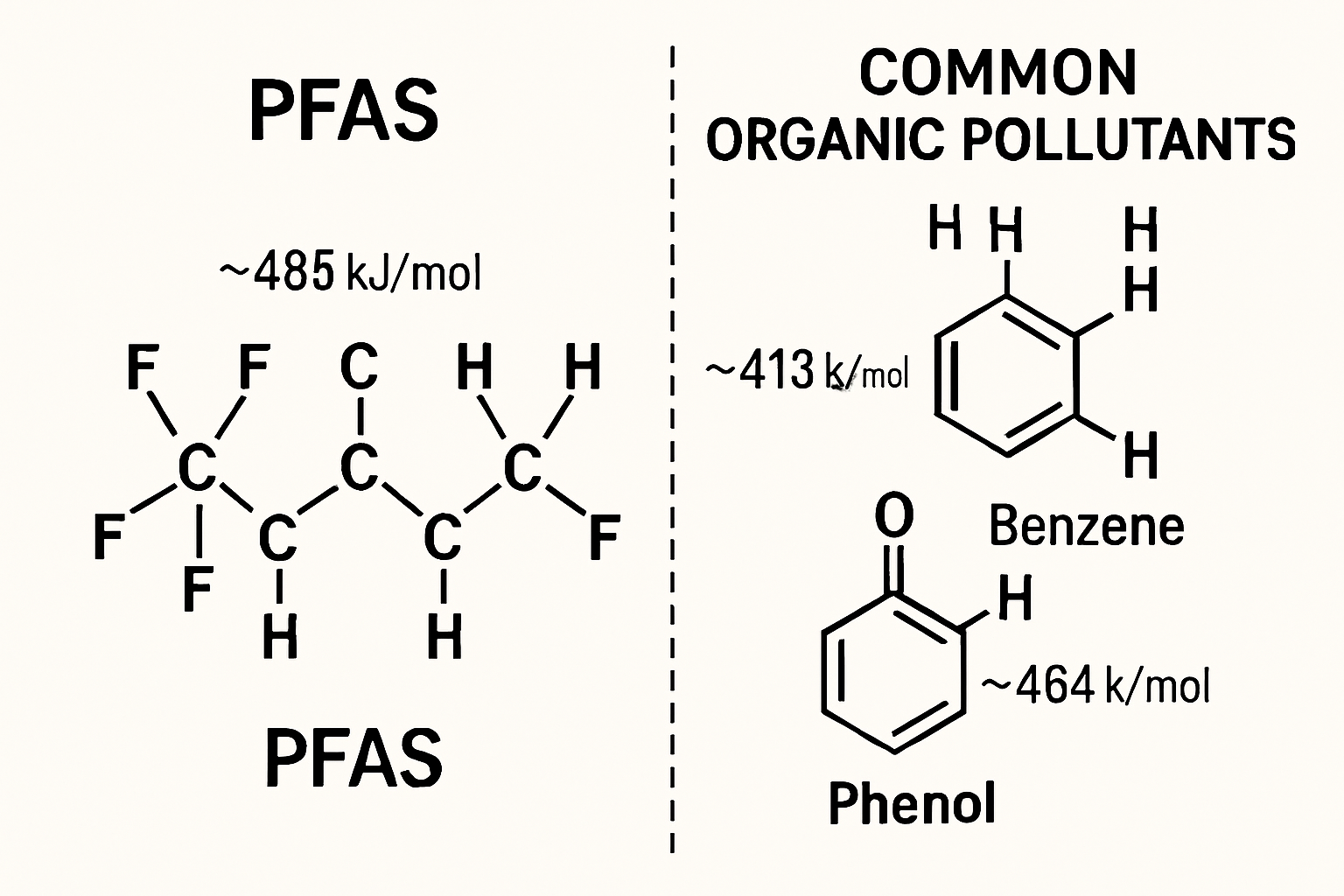
Per- and polyfluoroalkyl substances (PFAS) represent a class of thousands of synthetic chemicals, globally recognized as "forever chemicals" due to the immense strength of their carbon-fluorine (C-F) bonds, which makes them extraordinarily resistant to environmental and biological degradation. Their widespread use in industrial applications and consumer products has led to their ubiquitous presence in water sources worldwide, posing significant risks to ecosystems and human health. Conventional water treatment methods, such as activated carbon adsorption and reverse osmosis, are non-destructive, merely transferring PFAS from water to another medium, creating a secondary waste problem and necessitating costly disposal or regeneration. There is a critical and urgent need for destructive technologies that can mineralize these persistent pollutants into benign compounds.
This article proposes a novel and speculative approach: Magneto-Acoustic Catalysis. This strategy synergistically combines the physical power of high-frequency ultrasound (sonochemistry) with the catalytic activity of engineered magnetic nanoparticles. The core hypothesis is that by integrating these two advanced oxidation processes, we can create a highly efficient and recoverable system capable of breaking the resilient C-F bond. The acoustic field generates intense, localized hotspots through cavitation, while the magnetic nanoparticles act as both catalysts and recoverable seeds for this cavitation, enhancing the production of reactive radical species and creating a powerful, targeted degradation zone for PFAS molecules adsorbed on their surface. This integrated approach offers a promising pathway to a scalable and sustainable solution for the remediation of PFAS-contaminated waterways.
The Inertia of the Carbon-Fluorine Bond and the Limits of Current Technologies
The chemical stability of PFAS is rooted in the C-F bond, which is one of the strongest single bonds in organic chemistry, with a bond dissociation energy of over 100 kcal/mol. This inherent strength makes PFAS compounds highly resistant to chemical, thermal, and biological degradation pathways that effectively break down other organic pollutants. As a result, they persist and bioaccumulate, leading to widespread and long-lasting contamination of soil, groundwater, and surface water. Concerns over their health effects, including a range of cancers, immune system dysfunction, and developmental issues, have led to increasingly stringent regulatory advisories, as noted in studies of the Delaware River watershed (Akbari et al., 2025).
Current remediation technologies are largely insufficient for complete destruction. Adsorption methods using materials like granular activated carbon or, more recently, advanced materials like covalent organic frameworks (Issaka et al., 2024; Liu et al., 2022), are effective at concentrating PFAS but do not destroy them. Thermal technologies like incineration require extremely high temperatures and are energy-intensive, with risks of incomplete combustion and the release of other hazardous byproducts. Therefore, the development of destructive technologies that can operate at ambient temperature and pressure is a primary goal in environmental engineering (Tshangana et al., 2025).
Sonochemical Degradation: Using Sound to Break the Unbreakable
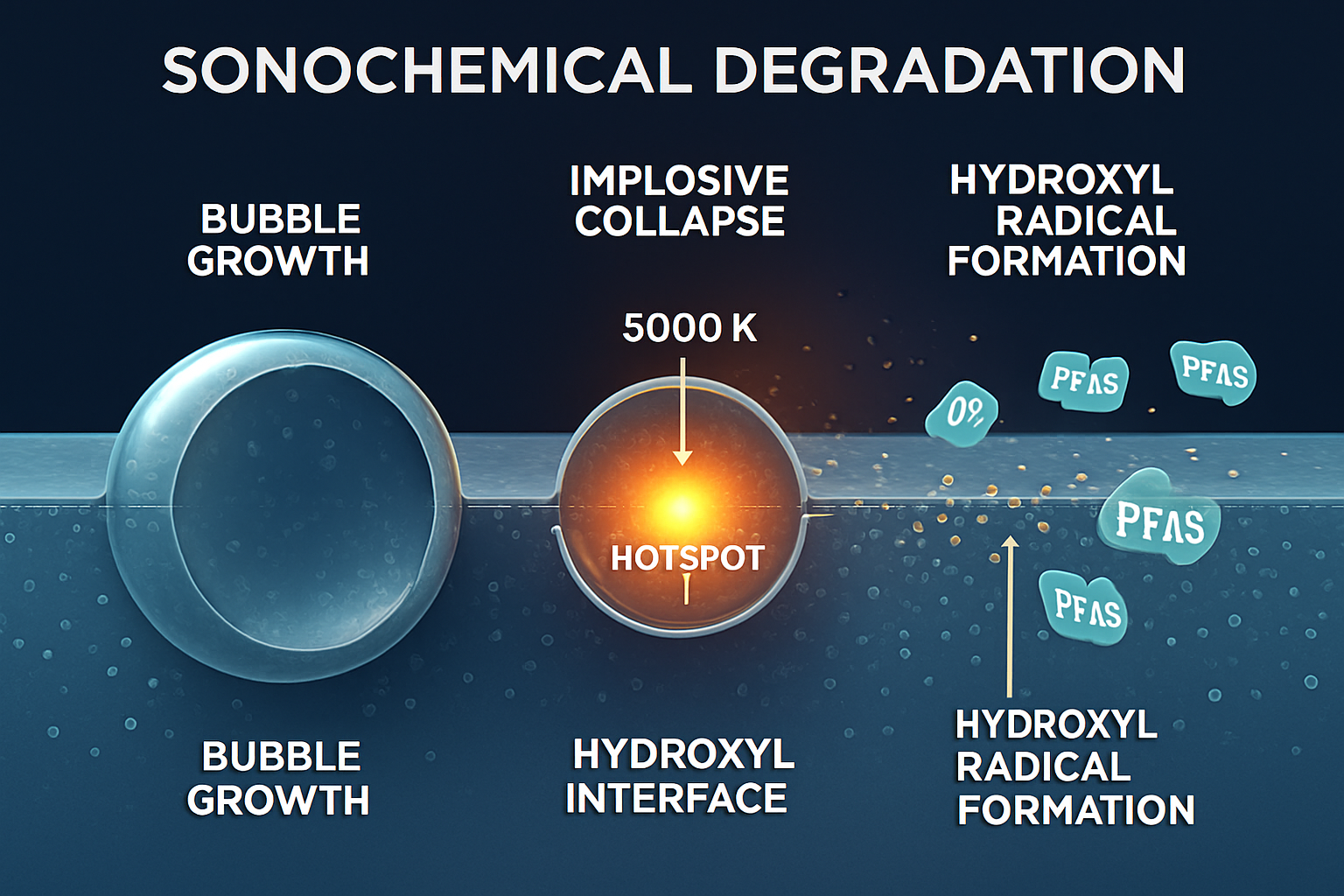
Sonochemistry utilizes the energy of high-frequency ultrasound (typically >20 kHz) to induce acoustic cavitation in a liquid medium. This process involves the formation, growth, and violent collapse of microscopic bubbles. The implosion of these bubbles is an adiabatic process, creating transient, localized "hotspots" with temperatures reaching over 5000 K, pressures up to 1000 atm, and colossal heating and cooling rates. These extreme conditions lead to two primary mechanisms for pollutant degradation: pyrolytic decomposition of molecules within the collapsing bubble, and the generation of highly reactive radical species, primarily hydroxyl radicals (•OH), from the homolytic fission of water molecules at the bubble-liquid interface.
While the direct pyrolysis of PFAS is possible under these conditions, the accumulation of these amphiphilic molecules at the bubble interface makes them prime targets for radical attack. The generated •OH, along with other reactive oxygen species (ROS), can initiate the cleavage of the C-F bonds—a critical step in the degradation pathway. Studies have demonstrated the viability of sonocatalytic processes for degrading complex organic molecules like dyes (Rodríguez-Flores et al., 2024), indicating the powerful oxidative environment created by ultrasound. However, the efficiency of sonolysis alone for PFAS remediation can be limited by the energy-intensive nature of ultrasound and the need to enhance radical production.
Magnetic Nanoparticles as Recoverable Catalysts
In recent years, magnetic nanoparticles—particularly those based on iron oxides (e.g., magnetite, Fe3O4) and zero-valent iron (ZVI)—have emerged as highly effective catalysts for advanced oxidation processes. Their high surface-area-to-volume ratio provides abundant active sites for catalytic reactions. Iron-based nanoparticles are especially effective in Fenton-like reactions, catalyzing the decomposition of oxidants like hydrogen peroxide to produce powerful hydroxyl radicals for pollutant degradation (Lee et al., 2025). A key advantage is their magnetic nature, allowing rapid and efficient separation from water using external magnets. After treatment, the nanoparticles can be recovered, regenerated, and reused, reducing operational costs and minimizing nanoparticle release into the environment—addressing a major drawback of other nano-catalytic systems (Meng et al., 2024).
This feature makes magnetic nanoparticles critical for sustainable, economically viable treatment technologies. Continued innovation in nanoparticle composition, size, and surface chemistry promises to enhance their catalytic potential, efficiency, and environmental safety. Emerging research also explores core-shell structures and composites, such as iron biochar, to further improve PFAS removal and treatment dynamics (Masud et al., 2025).

The Proposed Synthesis: A Magneto-Acoustic Synergy for PFAS Destruction
The central proposition is that combining sonochemistry and magnetic catalysis will create a synergistic effect far more powerful than the sum of its parts. This "magneto-acoustic catalysis" relies on several interconnected mechanisms:
- Enhanced Cavitation Nucleation: Magnetic nanoparticles serve as heterogeneous nuclei for cavitation bubbles, increasing the frequency and distribution of cavitation events and thus the overall efficiency of sonochemical degradation.
- Localized Catalytic Hotspots: Bubble collapse occurs at nanoparticle surfaces, generating extreme temperatures and pressures directly at the catalytic surface. PFAS molecules adsorbed on these surfaces undergo thermal stress, shockwaves, and intense radical exposure, accelerating degradation rates.
- Acoustic Surface Cleaning and Activation: Cavitation-generated micro-jets and shockwaves continually clean nanoparticle surfaces, preventing fouling and maintaining high catalytic efficiency throughout multiple operational cycles.
- Mechanocatalytic Enhancement: Physical forces during cavitation collapse induce lattice strain and create surface defects on nanoparticles, producing new highly reactive catalytic sites in a process known as mechanocatalysis. The synergy between mechanical stress and catalytic function further boosts degradation efficiency (Fazli et al., 2022).
An external magnetic field can further manipulate and recover the nanoparticles during and after treatment, offering spatial control and sustainability for practical deployment in real-world contaminated water systems.
Conclusion
Magneto-acoustic catalysis represents a frontier in environmental remediation, with the potential to deliver a highly effective solution to the challenge of persistent PFAS pollution. Merging sonochemical cavitation with the catalytic reactivity of magnetic nanoparticles establishes a dual-action platform capable of breaking C-F bonds in situ and at scale. The recoverable and reusable nature of the catalyst system addresses sustainability and operational cost issues, making the approach well-suited for continuous water remediation.
Further research should focus on optimizing nanoparticle composition, ultrasound frequency and power, and the interaction parameters governing the synergy. Exploring hybrid catalysts and tailored magnetic fields can yield greater performance and control. While challenges remain in scaling up the technology, magneto-acoustic catalysis offers a compelling direction for breaking down "forever chemicals" and restoring water quality for communities worldwide.
References
- Akbari, E. et al. (2025). Per- and Polyfluoroalkyl Substances (PFAS) in Urbanized Section of the Delaware River Watershed: Risk Assessment and Geographical Distribution. Water, Air, & Soil Pollution. https://doi.org/10.1007/s11270-025-07835-0
- Fathy, N. et al. (2025). Acid blue 40 dye decolorization using magnetite nanoparticles with reduced graphene oxide and mesoporous silica as Fenton catalysts. Scientific Reports. https://doi.org/10.1038/s41598-025-91382-5
- Fazli, A. et al. (2022). A BaTiO3/WS2 composite for piezo-photocatalytic persulfate activation and ofloxacin degradation. Communications Chemistry. https://doi.org/10.1038/s42004-022-00707-2
- Issaka, E. et al. (2024). Covalent organic frameworks: a review of synthesis methods, properties and applications for per- and poly-fluoroalkyl substances removal. Clean Technologies and Environmental Policy. https://doi.org/10.1007/s10098-024-03102-8
- Lee, J. et al. (2025). Microwave-enhanced catalytic degradation of organic compounds with silica-coated iron oxide nanocrystals via fenton-like reaction pathway. npj Clean Water. https://doi.org/10.1038/s41545-025-00449-3
- Liu, X. et al. (2022). Installation of synergistic binding sites onto porous organic polymers for efficient removal of perfluorooctanoic acid. Nature Communications. https://doi.org/10.1038/s41467-022-29816-1
- Masud, M. A. A. et al. (2025). Iron biochar synergy in aquatic systems through surface functionalities electron transfer and reactive species dynamics. npj Clean Water. https://doi.org/10.1038/s41545-025-00471-5
- Meng, H. et al. (2024). Magnetic hydrochar for sustainable wastewater management. npj Materials Sustainability. https://doi.org/10.1038/s44296-024-00047-3
- Moreira, R. et al. (2024). Hybrid graphenic and iron oxide photocatalysts for the decomposition of synthetic chemicals. Communications Engineering. https://doi.org/10.1038/s44172-024-00267-4
- Rodríguez-Flores, T. et al. (2024). Sonocatalytic degradation of RB-5 dye using ZnO nanoparticles doped with transition metals. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-024-35776-4
- Tshangana, C. S. et al. (2025). Technology status to treat PFAS-contaminated water and limiting factors for their effective full-scale application. npj Clean Water. https://doi.org/10.1038/s41545-025-00457-3