Liquid Metal Catalysis for CO2 Conversion into Value-Added Chemicals
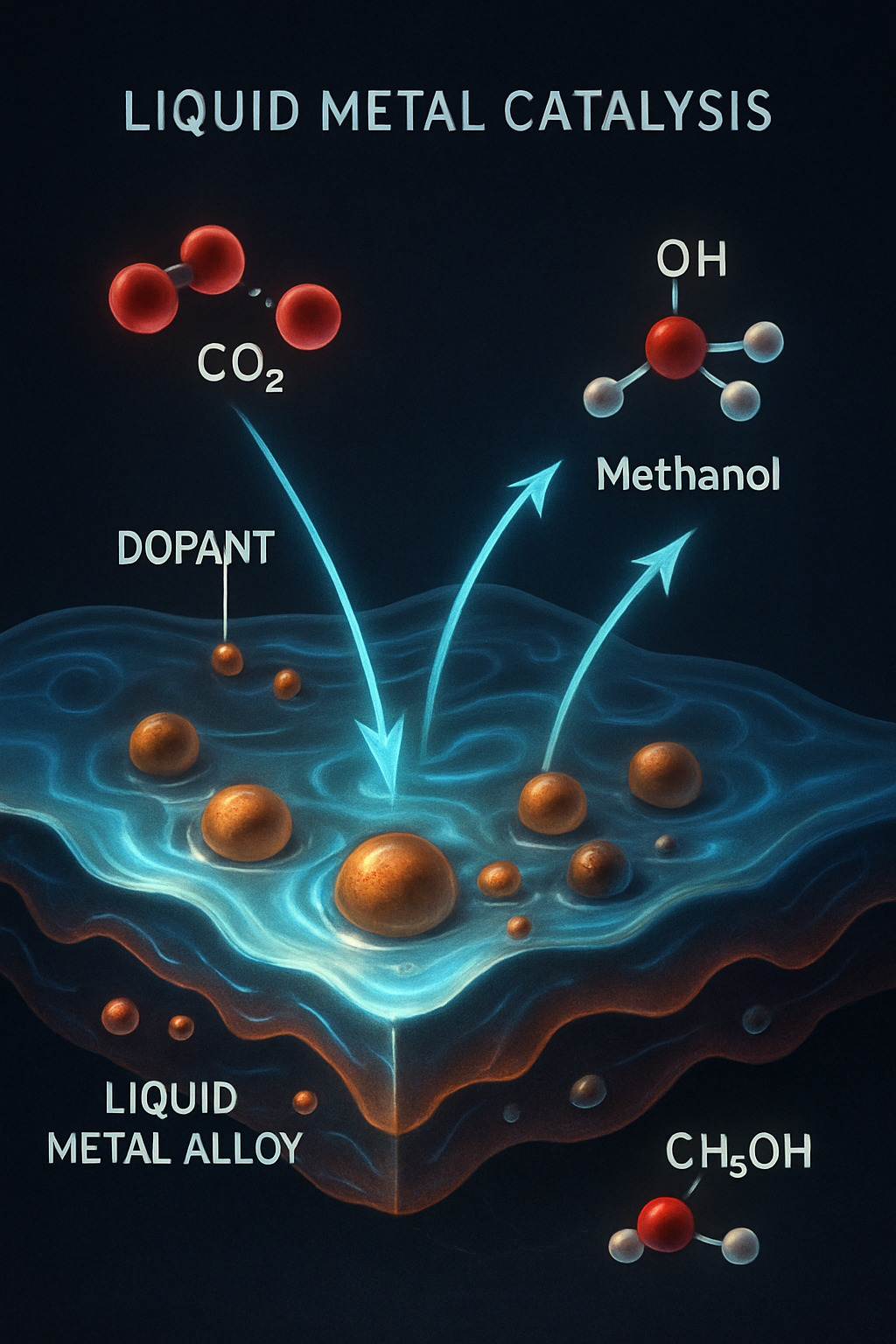
Liquid metal catalysis, particularly involving gallium (Ga) and indium (In) based alloys, is a rapidly developing field presenting revolutionary possibilities for the sustainable conversion of carbon dioxide (CO2) into valuable chemicals. These innovative catalytic systems offer promising approaches to reducing greenhouse gas emissions while establishing new pathways for green chemical production. The unique properties of liquid metals—such as their tunable solubility, high CO2 affinity, and the ability to operate under mild conditions—distinguish them from conventional solid-state catalysts and drive interest in their scientific and industrial applications.
In this article, we explore breakthroughs in liquid metal-based CO2 catalysis, focusing on gallium and indium systems, discuss mechanistic insights, and outline the challenges and opportunities for future implementation.
Gallium-Based Liquid Metal Catalysts
A significant advantage of liquid metal catalysts is their capacity to activate and convert CO2 at relatively low temperatures—substantially lower than those required for traditional thermocatalysis. Gallium-based liquid metals, often combined with dopants or alloying elements, have demonstrated potential in both photocatalytic degradation of pollutants and direct CO2 utilization. For example, gallium-rich supported catalytically active liquid metal solutions (SCALMS) have undergone extensive investigation for dry reforming of methane—a process integrally related to CO2 utilization. Although some systems succumb to oxidation, the judicious selection of alloying elements, such as nickel (Ni), and optimal process conditions can overcome these stability challenges. Ga-Ni SCALMS exhibit activity based on a kinetically regulated redox process (Wolf et al., 2023).
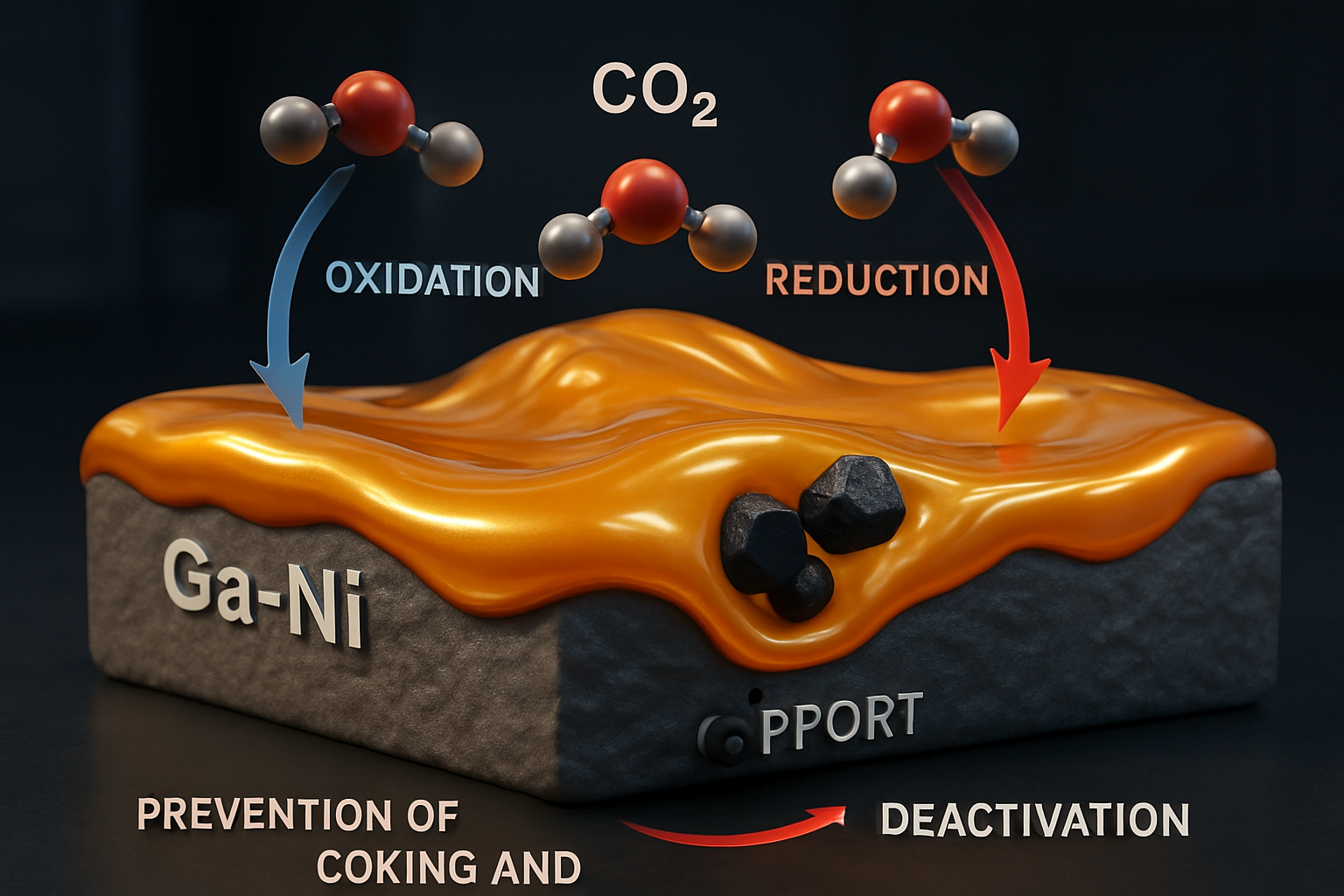
The use of liquid metals in electrocatalytic systems is also gaining traction. These catalysts hold promise for the electrochemical conversion of CO2 to formic acid and other C1 chemicals, with the fluid phase helping to mitigate issues like coking and deactivation often associated with their solid counterparts.
Indium-Based Liquid Metal Catalysts and Derivatives
Indium-based systems are prominent contenders in the arena of CO2 catalytic conversion. Indium-containing metal–organic frameworks (MOFs) have emerged as efficient photocatalysts and electrocatalysts. For instance, In-MOF/graphene oxide heterostructures maintain robust CO2 reduction activity even in oxygen-rich environments (Zhang et al., 2024). Another avenue involves In2O3-based catalysts—sometimes promoted with palladium—that deliver potent activity and selectivity for the hydrogenation of CO2 to methanol (Stangeland et al., 2020; Frei et al., 2019).
These remarkable properties are largely attributed to indium's electronic structure and the presence of oxygen vacancy-rich surfaces, which facilitate efficient CO2 activation and conversion. Modifying the catalyst composition and architecture allows fine-tuning of product selectivity and efficiency. Such tunability, combined with high Faradaic efficiency in electrocatalytic systems, makes indium derivatives an area of strong research focus.

Photothermal and Photoelectrocatalytic Approaches
Another highly promising domain is the photothermal and photoelectrocatalytic conversion of CO2, sometimes incorporating liquid metal components or their derivatives. These solar-driven systems are designed to mimic key aspects of natural photosynthesis, and nanocatalysts featuring liquid metal-like properties are integral to their function (Cheng et al., 2025). At the heart of these systems are plasmonic effects, where metallic nanoparticles boost light absorption and create hot electrons capable of driving CO2 reduction at the liquid metal surface. This provides a pathway to couple CO2 conversion with renewable energy sources.
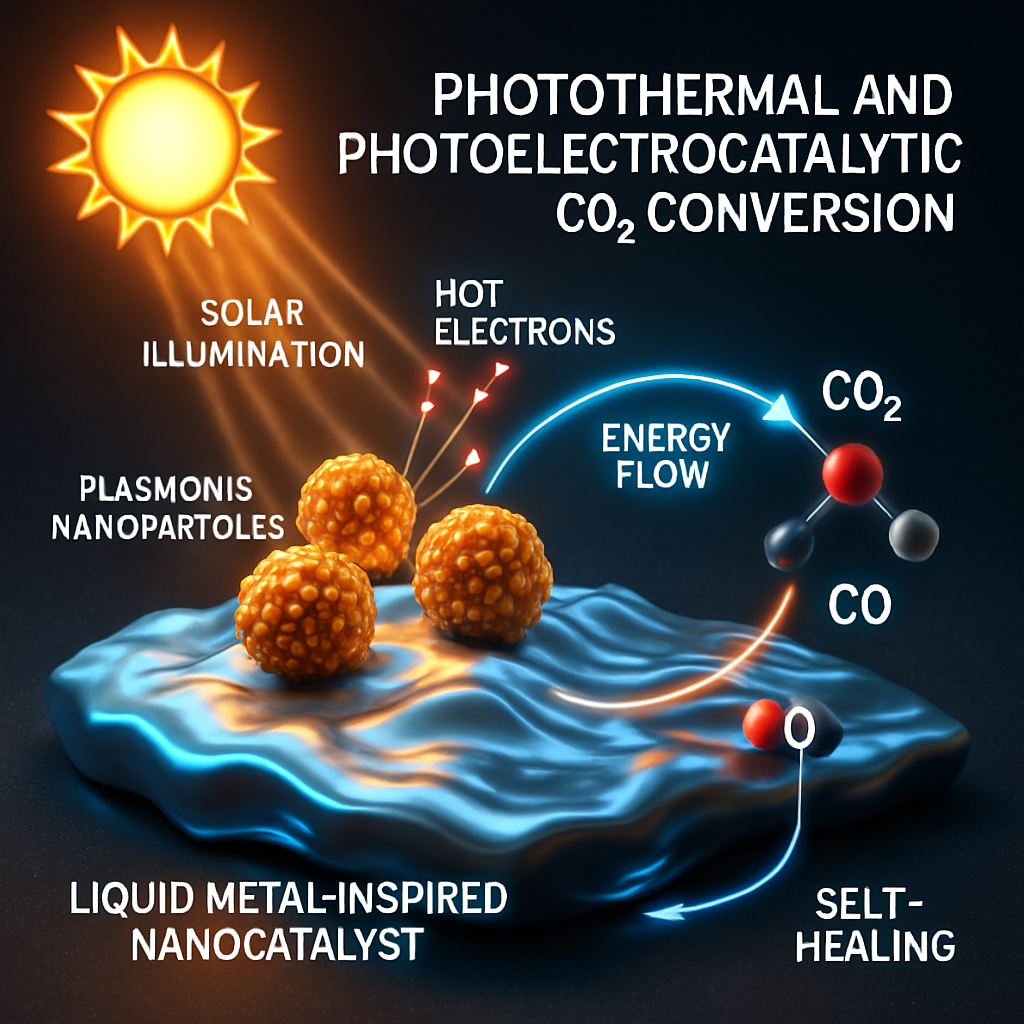
Critical hurdles for these approaches include ensuring the stability and durability of catalysts under intense illumination and fluctuating conditions. However, the potential of liquid metal surfaces for self-healing and regeneration offers significant advantages in overcoming deactivation and prolonging catalyst lifetime, making them attractive for integrated solar conversion technologies.
Challenges and Future Directions
Despite the clear promise of liquid metal catalysis for CO2 conversion, several hurdles must be overcome to realize their widespread deployment. Chief among these are issues of long-term stability, particularly under oxidative or high-temperature conditions, and the potential for leaching of active components. Additionally, a more nuanced mechanistic understanding is necessary—particularly regarding the atomic-level interaction between CO2 and the dynamic liquid metal surface, the formation and stabilization of intermediates, and control over product selectivity (Ye et al., 2019).
Advanced theoretical modeling, such as density functional theory (DFT) combined with sophisticated in-situ and operando experimental techniques, will be pivotal in unraveling these mechanisms. Recent innovations, such as engineered bismuth (Bi) catalysts with tensile strain, provide new approaches to activating previously inert sites (Chen et al., 2025). Achieving industrial-scale applications will also require the development of robust reactors, innovative product separation strategies, and possibly manufacturing advances like 3D-printed electrodes (Wang et al., 2025). The pursuit of co-hydrogenation of CO and CO2, as well as hybrid material systems, further underscores the vast scope and ambition of research in this area (Li et al., 2025).
Conclusion
Liquid metal catalysis stands as a novel and compelling technological frontier in the pursuit of sustainable CO2 conversion and valorization. Gallium and indium-based systems, integrating properties such as high tunability, mild operational conditions, and inherent self-healing behavior, are catalyzing new avenues for chemical production derived from greenhouse gases. Nevertheless, progress toward commercial realization depends on addressing stability, in-depth mechanistic elucidation, and scalable process engineering.
Continued interdisciplinary research efforts, combining catalyst innovation with advanced characterization and process design, will be essential for unlocking the potential of liquid metal catalysts. The synergistic integration of solar energy, hybrid material strategies, and dynamic liquid metal interfaces is expected to drive the development of efficient, circular carbon technologies for the future.
References
- Chen, X., Lu, R., Li, C., Luo, W., Yu, R., Zhu, J., Lv, L., Dai, Y., Gong, S., Zhou, Y., Xiong, W., Wu, J., Cai, H., Wu, X., Deng, Z., Xing, B., Su, L., Wang, F., Chao, F., Chen, W., Xia, C., Wang, Z., & Mai, L. (2025). Activating inert non-defect sites in Bi catalysts using tensile strain engineering for highly active CO2 electroreduction. Nature Communications. https://doi.org/10.1038/s41467-025-56975-8
- Cheng, Q., Wang, L., & Huang, J. (2025). Solar-driven nanocatalysts for CO2 upcycling: the structure-performance correlation. Carbon Neutral Systems. https://doi.org/10.1007/s44438-025-00005-z
- Frei, M. S., Mondelli, C., García-Muelas, R., Kley, K. S., Puértolas, B., López, N., Safonova, O. V., Stewart, J. A., Curulla Ferré, D., & Pérez-Ramírez, J. (2019). Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation. Nature Communications. https://doi.org/10.1038/s41467-019-11349-9
- Li, K., Cai, J., Wang, Y., Cheng, X., Li, D., Li, Z., Zhao, H., Tian, D., Zhu, T., & Wang, H. (2025). Co-hydrogenation of CO2 and CO to methanol: a perspective. Carbon Neutral Systems. https://doi.org/10.1007/s44438-025-00006-y
- Orozco, S., Martínez-Aguilar, E., Belver, C., Bedia, J., & Rivero, M. (2025). Simulation and experimentation of iron-doped liquid metal-based gallium oxide photocatalysts for environmental applications harnessing solar energy. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-025-36436-x
- Qiu, C., Qian, K., Yu, J., Sun, M., Cao, S., Gao, J., Yu, R., Fang, L., Yao, Y., Lu, X., Li, T., Huang, B., & Yang, S. (2022). MOF-Transformed In2O3-x@C Nanocorn Electrocatalyst for Efficient CO2 Reduction to HCOOH. Nano-Micro Letters. https://doi.org/10.1007/s40820-022-00913-6
- Stangeland, K., Li, H., & Yu, Z. (2020). CO2 hydrogenation to methanol: the structure–activity relationships of different catalyst systems. Energy, Ecology and Environment. https://doi.org/10.1007/s40974-020-00156-4
- Wang, W., Zhao, N., Zhao, K., Zhang, M., Pang, K., Zhang, Y., & Yuan, J. (2025). Multi-heteroatom-doped porous carbon electrodes from 3D printing and conformal carbonization of ionic liquids for electrocatalytic CO2 conversion into syngas. Communications Chemistry. https://doi.org/10.1038/s42004-025-01514-1
- Wolf, M., Oliveira, A. L., Taccardi, N., Maisel, S., Heller, M., Khan Antara, S., Søgaard, A., Felfer, P., Görling, A., Haumann, M., & Wasserscheid, P. (2023). Dry reforming of methane over gallium-based supported catalytically active liquid metal solutions. Communications Chemistry. https://doi.org/10.1038/s42004-023-01018-w
- Yang, Y., Liu, X., He, D., & Jin, F. (2025). 100% Conversion of CO2–CH4 with Non-Precious Co@ZnO Catalyst in Hot Water. Nano-Micro Letters. https://doi.org/10.1007/s40820-025-01711-6
- Ye, R.-P., Ding, J., Gong, W., Argyle, M. D., Zhong, Q., Wang, Y., Russell, C. K., Xu, Z., Russell, A. G., Li, Q., Fan, M., & Yao, Y.-G. (2019). CO2 hydrogenation to high-value products via heterogeneous catalysis. Nature Communications. https://doi.org/10.1038/s41467-019-13638-9
- Zhang, Z., Wang, Y., Xie, Y., Tsukamoto, T., Zhao, Q., Huang, Q., Huang, X., Zhang, B., Song, W., Chen, C., Sheng, H., & Zhao, J. (2024). Floatable artificial leaf to couple oxygen-tolerant CO2 conversion with water purification. Nature Communications. https://doi.org/10.1038/s41467-024-55753-2