Harnessing Hydrothermal Vent Microbiomes: Syntrophy and Methanogenesis for Industrial Biotechnology
Deep-sea hydrothermal vents represent some of the most extreme environments on Earth, characterized by high temperatures, immense pressure, darkness, and unique geochemistry rich in reduced compounds like hydrogen sulfide, methane, and hydrogen. Despite these harsh conditions, vents host thriving microbial ecosystems dominated by chemolithoautotrophic Archaea and Bacteria. These microorganisms have evolved unique metabolic strategies to harness geochemical energy, forming the base of the local food web. Among the most fascinating aspects of these microbiomes are the intricate syntrophic interactions, where different microbial species cooperate metabolically, often enabling processes that are thermodynamically unfavourable for individual species.
This article explores the potential of harnessing these unique microbial consortia, particularly those involving syntrophy and methanogenesis, for industrial biotechnology. Hydrothermal vent microorganisms, being extremophiles, often possess robust enzymes and metabolic pathways that function under extreme conditions, making them attractive candidates for various industrial applications. Understanding the syntrophic relationships, especially those leading to methane production (methanogenesis), could unlock novel biotechnological processes for energy generation, bioremediation, and the production of valuable biochemicals.
The Hydrothermal Vent Microbiome - An Extreme Environment
Hydrothermal vents are geological phenomena where geothermally heated water discharges from the seafloor. The vent fluids are typically anaerobic and enriched in dissolved minerals, metals, and gases (e.g., H2S, CH4, H2, CO2), creating steep chemical and thermal gradients where they mix with cold, oxygenated seawater. These conditions select for extremophilic microorganisms, primarily hyperthermophiles and thermophiles, piezophiles (pressure-loving), and chemolithoautotrophs capable of deriving energy from inorganic chemical reactions rather than sunlight.
The microbial diversity in these ecosystems is vast and includes many novel lineages from both the Archaea and Bacteria domains. Key metabolic processes include sulfide oxidation, sulfate reduction, hydrogen oxidation, iron oxidation/reduction, and methanogenesis. Many of these organisms represent deep branches in the tree of life, offering insights into early life evolution and the limits of life on Earth. Their unique adaptations, such as thermostable enzymes and specialized metabolic pathways, are of significant interest for biotechnological exploration.
Recent advances in cultivation-independent techniques, like metagenomics and metatranscriptomics, have been crucial in exploring the functional potential of these largely uncultured communities. These studies reveal complex community structures and metabolic networks, highlighting the importance of microbial interactions, such as syntrophy, in sustaining these unique ecosystems. Understanding the ecology and physiology of these extremophiles is the first step towards harnessing their potential.
Syntrophy in Deep-Sea Vents

Syntrophy, meaning "feeding together," is a specialized form of mutualism where two or more microorganisms cooperate to degrade a substrate that neither can utilize alone. This metabolic coupling is crucial in anaerobic environments like hydrothermal vents, particularly for processes that are near the thermodynamic equilibrium limit. A common form involves interspecies hydrogen transfer (IHT), where hydrogen gas (H2), produced as a metabolic byproduct by one organism (e.g., during fermentation or anaerobic oxidation), is consumed by another (e.g., methanogens, sulfate reducers).
In hydrothermal vent sediments and plumes, syntrophic interactions are vital for the anaerobic oxidation of methane (AOM) and the degradation of complex organic matter or hydrocarbons, if present. For example, consortia of anaerobic methane-oxidizing archaea (ANME) and sulfate-reducing bacteria (SRB) couple methane oxidation to sulfate reduction. Similarly, hydrogenotrophic methanogens often rely on hydrogen-producing syntrophic partners that ferment organic compounds or anaerobically oxidize substrates like short-chain fatty acids or alcohols.
These syntrophic partnerships drive key biogeochemical cycles (carbon, sulfur, nitrogen) within the vent ecosystem. The close physical association often observed in syntrophic consortia, sometimes forming structured aggregates or biofilms, facilitates efficient metabolite exchange. Unraveling these partnerships, often involving uncultured microbes, requires sophisticated molecular and microscopic techniques combined with thermodynamic modelling, but is essential for understanding ecosystem function and identifying potential biotechnological targets.
Methanogenesis in Hydrothermal Vents
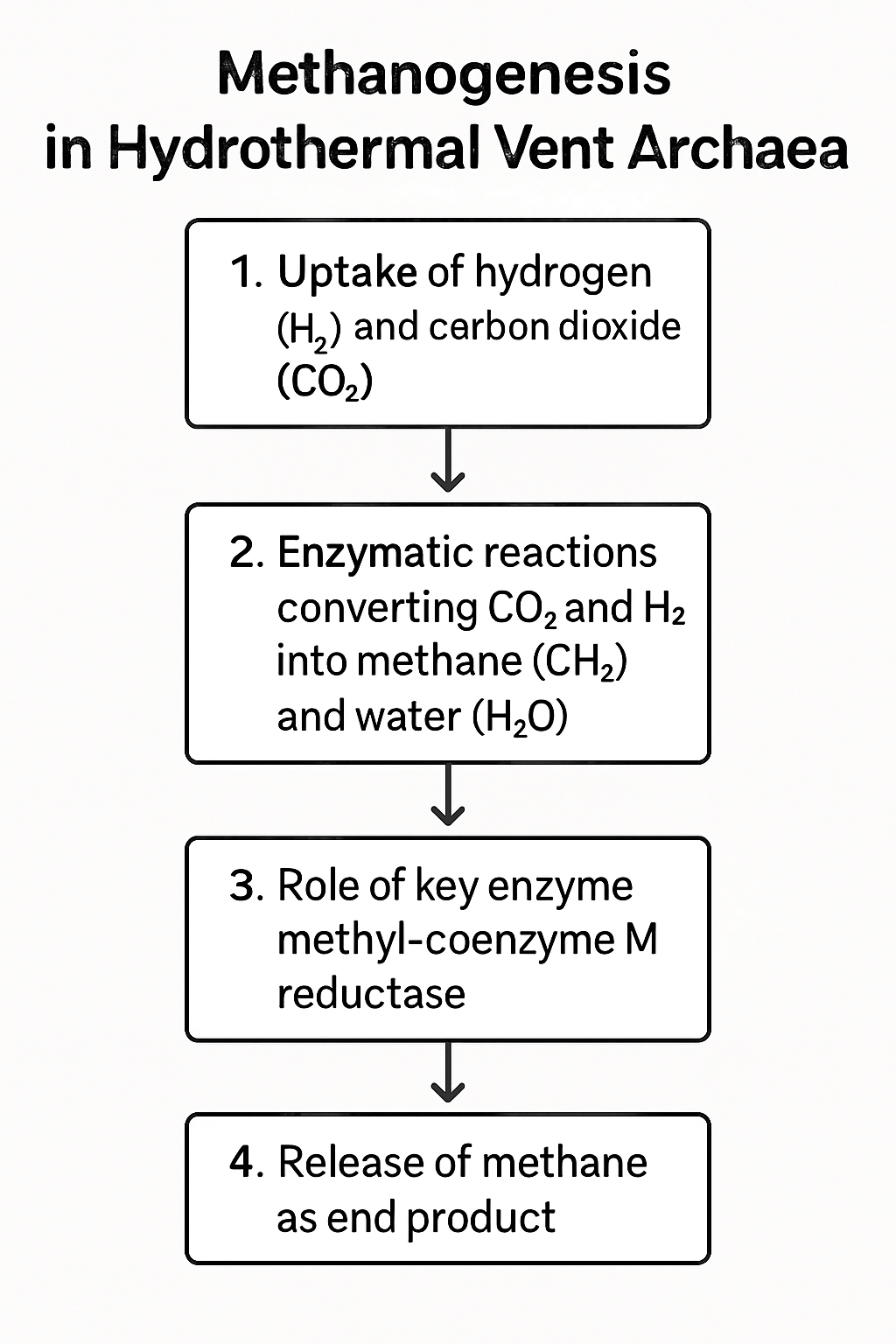
Methanogenesis, the biological production of methane (CH4), is a key process in anaerobic environments and is exclusively carried out by methanogenic Archaea. In hydrothermal vents, methane can have both abiotic (serpentinization) and biotic origins. Biological methanogenesis provides an important energy sink, particularly utilizing hydrogen and carbon dioxide, which are often abundant in vent fluids. Hydrogenotrophic methanogenesis (4H2 + CO2 → CH4 + 2H2O) is considered a primary pathway in these systems, fueled by geochemically produced H2 or H2 generated by syntrophic partners.
Several genera of thermophilic and hyperthermophilic methanogens, such as Methanocaldococcus, Methanothermococcus, and Methanopyrus, are commonly found in hydrothermal vents. These organisms thrive at high temperatures and pressures, possessing unique enzymes and metabolic adaptations. Their activity is often tightly coupled with hydrogen-producing microorganisms through syntrophy. For example, the anaerobic degradation of organic matter or the oxidation of certain inorganic compounds by bacteria or other archaea can generate the H2 necessary to fuel methanogenesis.
The efficiency of methanogenesis in these environments is often limited by the availability of electron donors like H2. Syntrophic relationships overcome this limitation by maintaining low partial pressures of H2, making the H2-producing reactions thermodynamically favorable while supplying the methanogens with their required substrate. Studying these methanogenic consortia provides insights into potential strategies for enhancing biogas production under challenging industrial conditions, such as high temperature or salinity.
Industrial Biotechnology Potential

Hydrothermal vent microbiomes, particularly syntrophic consortia involving methanogens, offer significant potential for industrial biotechnology. The extremophilic nature of these organisms means their enzymes and metabolic pathways are often robust and active under conditions (e.g., high temperature, pressure, pH extremes, salinity) that would denature components from mesophilic organisms. This inherent stability is highly desirable for industrial processes, reducing the need for cooling and potentially increasing reaction rates.
One major application lies in enhanced biogas (methane) production. Thermophilic or hyperthermophilic anaerobic digestion using vent-derived methanogens or consortia could process recalcitrant feedstocks or operate at higher temperatures, potentially increasing efficiency and throughput. Syntrophic consortia could be engineered or optimized for specific waste streams, coupling breakdown of complex polymers or recalcitrant organics with efficient methanogenesis. Furthermore, the study of methanogenesis under high pressure could inform strategies for in situ biogas production in deep geological repositories or during subsurface resource extraction.
Beyond energy, these microbes are sources of novel biocatalysts. Thermostable enzymes (e.g., DNA polymerases, lipases, proteases, amylases) have revolutionized molecular biology and various industries. Syntrophic interactions might reveal novel enzymatic cascades or pathways for producing valuable chemicals or facilitating bioremediation of pollutants stable under extreme conditions, such as hydrocarbons or heavy metals. The ability of some vent microbes, including partners in syntrophy, to metabolize metals or sulfur compounds also points towards applications in biomining and bioremediation of industrial effluents. However, significant challenges remain in cultivating many of these extremophiles and scaling up processes from lab findings to industrial reality.
Conclusion
Deep-sea hydrothermal vents harbor unique microbial ecosystems driven by chemosynthesis and characterized by extremophilic adaptations and complex syntrophic interactions. The interplay between hydrogen producers and consumers, particularly hydrogenotrophic methanogens, is a cornerstone of energy flow in these environments. Harnessing these microbial consortia offers exciting prospects for industrial biotechnology, leveraging their robustness and unique metabolic capabilities for enhanced biogas production, discovery of novel enzymes, bioremediation, and potentially the synthesis of new biomaterials.
Future research should focus on integrating multi-omics approaches with advanced cultivation techniques and enrichment strategies to better understand and isolate key syntrophic partners and methanogens. Metabolic modelling and synthetic biology approaches could help in designing and optimizing microbial consortia for specific industrial applications. Overcoming the challenges associated with cultivating extremophiles and scaling up bioprocesses will be critical to translating the biotechnological potential of these fascinating deep-sea microbiomes into tangible industrial solutions.
References
- Knoflacher, M. (2024). Biological Energy Transformation. https://doi:10.1007/978-3-662-69423-7_7
- Noordam, S.J., Madjarov, J., Louro, R.O., & Paquete, C.M. (2024). Electroactive (Micro)organisms. https://doi:10.1007/978-3-031-54306-7_4
- Shu, W.-S., & Huang, L.-N. (2022). Microbial diversity in extreme environments. Nature Reviews Microbiology. https://doi:10.1038/s41579-021-00648-y
- Barton, L.L., & Fauque, G.D. (2022). Geomicrobiology, Biotechnology, and Industrial Applications. https://doi:10.1007/978-3-030-96703-1_7
- Pavlova, O.N., Izosimova, O.N., Chernitsyna, S.M., Ivanov, V.G., Pogodaeva, T.V., Khabuev, A.V., Gorshkov, A.G., & Zemskaya, T.I. (2022). Anaerobic oxidation of petroleum hydrocarbons in enrichment cultures from sediments of the Gorevoy Utes natural oil seep under methanogenic and sulfate-reducing conditions. Current Microbiology. https://doi:10.1007/s00248-021-01802-y
- Wang, Y., Kamagata, Y., Li, M., Han, F., Wang, F., & Xiao, X. (2021). New approaches for archaeal genome-guided cultivation. Science China Earth Sciences. https://doi:10.1007/s11430-020-9793-5
- Nakayama, C.R., Penteado, E.D., Duarte, R.T.D., Giachini, A.J., & Saia, F.T. (2019). Improved Methanogenic Communities for Biogas Production. https://doi:10.1007/978-3-030-10516-7_4
- Sar, P., Dutta, A., Bose, H., Mandal, S., & Kazy, S.K. (2019). Deep Biosphere: Microbiome of the Deep Terrestrial Subsurface. https://doi:10.1007/978-981-13-8315-1_8
- van Wolferen, M., Orell, A., & Albers, S.-V. (2018). Archaeal biofilm formation. Nature Reviews Microbiology. https://doi:10.1038/s41579-018-0058-4
- Stahl, D. A., Flowers, J. J., Hullar, M., & Davidson, S. (2013). Structure and Function of Microbial Communities. In The Prokaryotes (pp. 819-849). Springer Berlin Heidelberg. https://doi:10.1007/978-3-642-30123-0_34