Generative AI for De Novo Protein Design: From Latent Space to Laboratory

The ability to design proteins from first principles—'de novo' design—represents a grand challenge in biotechnology and medicine. For decades, scientists have sought to create bespoke proteins tailored for specific functions, from high-affinity therapeutics to novel industrial enzymes. This endeavor, however, has been profoundly difficult, limited by our incomplete understanding of the complex rules governing how a one-dimensional sequence of amino acids folds into a functional, three-dimensional structure.
Today, we stand at the precipice of a new era, powered by generative artificial intelligence. These advanced AI models, capable of learning the fundamental 'language' of proteins from vast datasets of known structures, are now generating entirely novel designs within the abstract computational realm of latent space. These are not mere modifications of existing scaffolds but truly original creations, conceived by an algorithm. This paradigm shift is rapidly closing the gap between computational concept and experimental validation, creating a direct pipeline from an AI's latent space to the laboratory bench and, as recent breakthroughs show, into clinical trials. The fusion of powerful generative architectures with rapid, AI-driven evaluation is not just accelerating protein engineering; it is fundamentally redefining the creative boundaries of the field.
The Generative Toolbox: From Denoising to Design
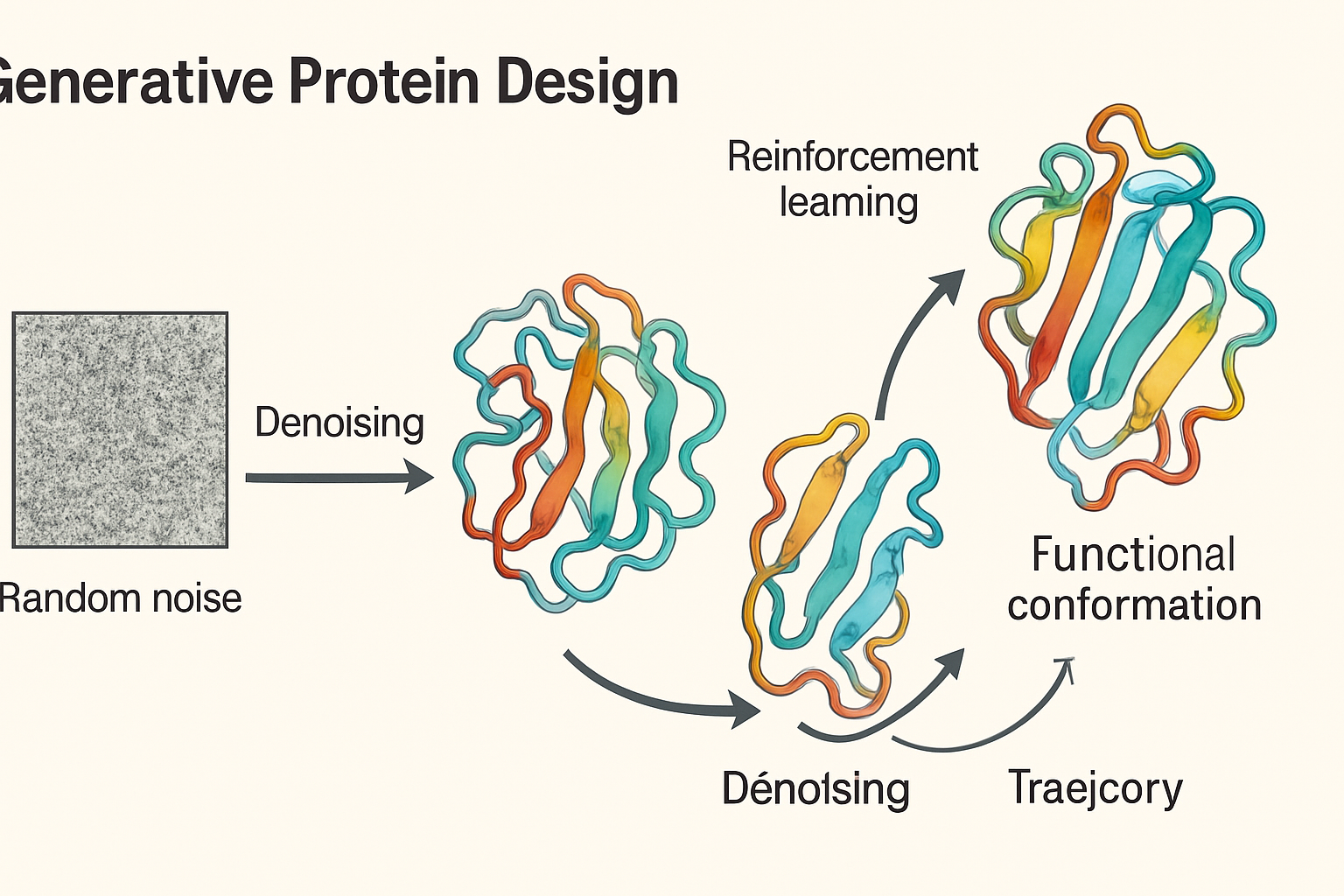
The engine driving this revolution is a class of generative models that has evolved significantly in recent years. While earlier approaches like Generative Adversarial Networks (GANs) showed promise, the state-of-the-art has shifted towards diffusion models. These models operate on a simple yet powerful principle: they learn to reverse a process of noise addition. A known protein structure is gradually corrupted into random noise, and the model is trained to reconstruct the original structure from that noisy state. By learning this 'denoising' process, the AI can then start with pure noise and 'sculpt' it into a coherent, physically plausible, and entirely novel protein structure. This an approach that has proven remarkably effective.
A critical enhancement to this process is the integration of Reinforcement Learning (RL). As described in recent frameworks for multi-target compound generation (Yuan, Y. et al., 2025), RL allows scientists to "steer" the generative process. The AI is rewarded for generating designs that meet specific, desirable criteria—such as predicted binding affinity to a target or optimal molecular properties. In this way, the diffusion model doesn't just generate random valid proteins; it actively searches the vast space of possibilities for designs that are optimized for a specific purpose. This combination of a powerful generative foundation (diffusion) with goal-oriented guidance (RL) creates a formidable tool for exploring chemical space for functional molecules.
The Critic in the Loop: The Generative-Critical Duality
A generated protein, no matter how elegant its design, is merely a hypothesis until it is tested. The traditional bottleneck in protein engineering has always been the slow, expensive process of wet-lab synthesis and testing. Here, a second AI revolution is taking place, creating what can be termed a 'Generative-Critical Duality'. For every AI model that acts as a 'generator,' another is being trained to be a 'critic.' These critic models are specialized predictors that can rapidly evaluate the generated designs in silico. A prime example is the development of machine learning models trained specifically to predict nanobody-antigen binding from structural features and energy scores (Shrestha, P. et al., 2025). Such tools serve as a high-throughput filter, assessing thousands of AI-generated candidates and identifying the small fraction most likely to succeed.
This duality—a generator proposing novel ideas and a critic efficiently vetoing the unpromising ones—creates an accelerated, iterative loop entirely within the computer. This cycle allows for the exploration of a vastly larger design space than would be possible with physical experiments alone. The most promising future direction for this paradigm is the tight integration of these two components, where the critic's feedback is used to directly refine the generator in real-time, creating a co-evolving system that gets progressively better at designing successful proteins. This synergy allows researchers to focus precious laboratory resources only on candidates that have already passed a rigorous virtual selection process, dramatically increasing the probability of success.

From Virtual Design to Clinical Reality: The Case of R-sertib
For years, the promise of AI-driven drug design has been largely theoretical. The ultimate proof lies in clinical validation. That milestone has now been reached. A recent Phase 2a clinical trial for idiopathic pulmonary fibrosis (IPF), a progressive and fatal lung disease, tested a drug named rentosertib (formerly ISM001-055). This molecule is a first-in-class inhibitor of TNIK, a novel target for IPF, and was discovered and designed using a generative AI platform (Xu, Z. et al., 2025). The successful trial, which demonstrated that the drug was safe and showed positive effects on lung function, represents a landmark achievement. It closes the loop, proving that a molecule conceived in the latent space of an AI can navigate the immense challenges of preclinical and clinical development to benefit patients.
The success of rentosertib is more than a single victory; it is a validation of the entire generative pipeline. It demonstrates that these AI systems can not only produce novel chemical matter but can do so in a way that satisfies the stringent requirements for safety, efficacy, and druggability. Furthermore, the knowledge gained from this trial—how the drug behaved in humans, its pharmacokinetic profile, and its side effects—provides invaluable data that can be fed back into the system. This creates a self-improving flywheel, where each clinical success (or failure) makes the next generation of AI models smarter, more accurate, and more likely to succeed. This ability to target previously 'undruggable' proteins and rapidly generate viable clinical candidates signals a profound shift in pharmaceutical R&D (Neelam, A. 2025).

Conclusion
Generative AI is no longer a speculative tool in protein engineering; it is a validated engine of innovation. By combining potent generative architectures like diffusion models with AI-powered critics for rapid evaluation, researchers have built a pipeline that leads from abstract bits in a computer to tangible molecules in the clinic. The journey of rentosertib from an AI's imagination to a successful Phase 2a trial is the watershed moment, proving the viability of this new paradigm. The challenge is no longer if AI can design novel proteins, but how we can best leverage this power.
Looking forward, the logical next step is the integration of these AI designers with fully automated, robotic wet labs, creating autonomous, closed-loop systems that can design, synthesize, and test new proteins with minimal human intervention. The complexity of the targets will also increase, moving from single-domain binders to sophisticated enzymes and dynamic molecular machines. However, to unlock the full potential of this technology, we must continue to address key challenges, including data quality and model interpretability (Olawade, D. B. et al., 2025). By striving to understand the principles behind the AI's successful designs, we may not only create new medicines and materials but also uncover new, fundamental rules of biology itself.
References
- Neelam, A. (2025). Advancing drug discovery: the role of computer-aided design and development in modern pharmaceuticals. Discover Pharmaceutical Sciences. https://doi.org/10.1007/s44395-025-00008-2
- Olawade, D. B. et al. (2025). Artificial Intelligence in Computational and Materials Chemistry: Prospects and Limitations. Chemistry Africa. https://doi.org/10.1007/s42250-025-01343-8
- Shrestha, P. et al. (2025). NanoBinder: a machine learning assisted nanobody binding prediction tool using Rosetta energy scores. Journal of Cheminformatics. https://doi.org/10.1186/s13321-025-01040-1
- Xu, Z. et al. (2025). A generative AI-discovered TNIK inhibitor for idiopathic pulmonary fibrosis: a randomized phase 2a trial. Nature Medicine. https://doi.org/10.1038/s41591-025-03743-2
- Yuan, Y. et al. (2025). A 3D generation framework using diffusion model and reinforcement learning to generate multi-target compounds with desired properties. Journal of Cheminformatics. https://doi.org/10.1186/s13321-025-01035-y