Electrocatalytic Upcycling of Plastic Waste into High-Value Monomers: A Circular Economy Approach
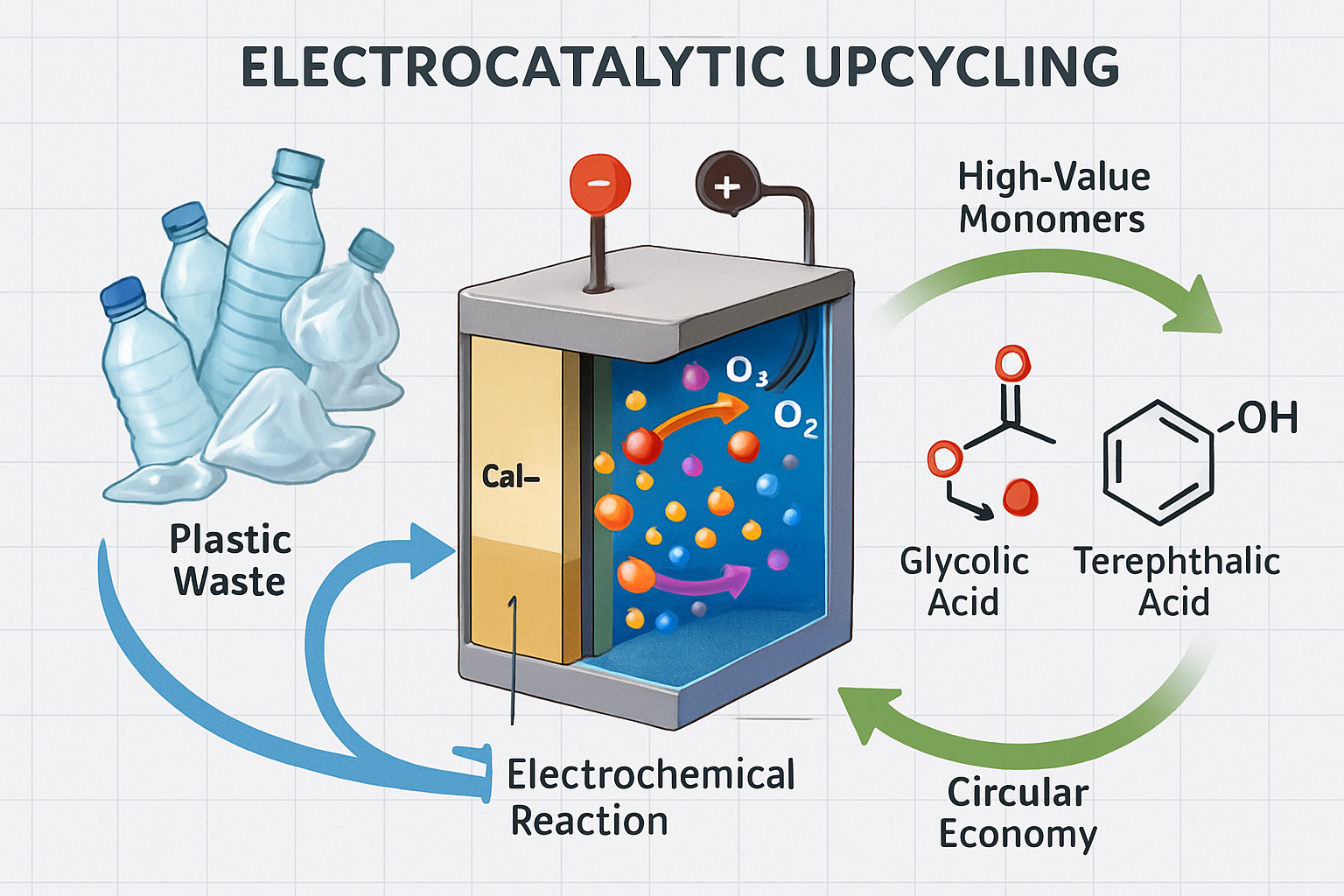
A severe global problem is plastic waste. Traditional recycling techniques frequently fall short of efficiently converting plastic waste into high-value products, resulting in resource depletion and environmental harm. Electrocatalysis has emerged as a viable technology for converting plastic waste into useful monomers, which is consistent with the circular economy's concepts. This method not only addresses plastic pollution but also provides a path for the creation of value-added chemicals from waste resources. However, the efficiency, selectivity, and scalability of electrocatalytic plastic upcycling require more research and development.
This article discusses recent breakthroughs in the electrocatalytic upcycling of plastic trash, with a particular emphasis on the conversion of common polymers such as polyethylene terephthalate (PET) into high-value monomers. It looks at different electrocatalytic systems, catalyst materials, and reaction conditions that improve product yield and selectivity. The importance of integrating these technologies into a circular economy framework is also emphasized, as is their potential to reduce reliance on virgin feedstocks and encourage sustainable chemical manufacturing. This study attempts to give a thorough overview of the current situation, problems, and future possibilities of electrocatalytic plastic valorization by combining results from diverse research.
Electrocatalytic Conversion of PET Waste
Polyethylene terephthalate (PET) is a widely used plastic that contributes considerably to plastic waste. Electrocatalytic upcycling of PET into useful monomers like glycolic acid (GA) and terephthalic acid (TPA) has shown considerable promise. Wang et al. (2025) created an interfacial acid-base microenvironment management technique for the efficient oxidation of PET-derived ethylene glycol (EG) to GA, reaching high current densities and a 93.0% GA selectivity in a pilot plant test. Similarly, Han et al. (2025) used pulsed electrocatalysis on a lamellar mesoporous PdCu (LM-PdCu) catalyst to achieve a GA Faraday efficiency of >92% and a yield rate of 0.475 mmol cm⁻² h⁻¹ for PET upcycling. These studies show the potential of electrocatalytic processes for selectively converting PET into important monomers under moderate circumstances.
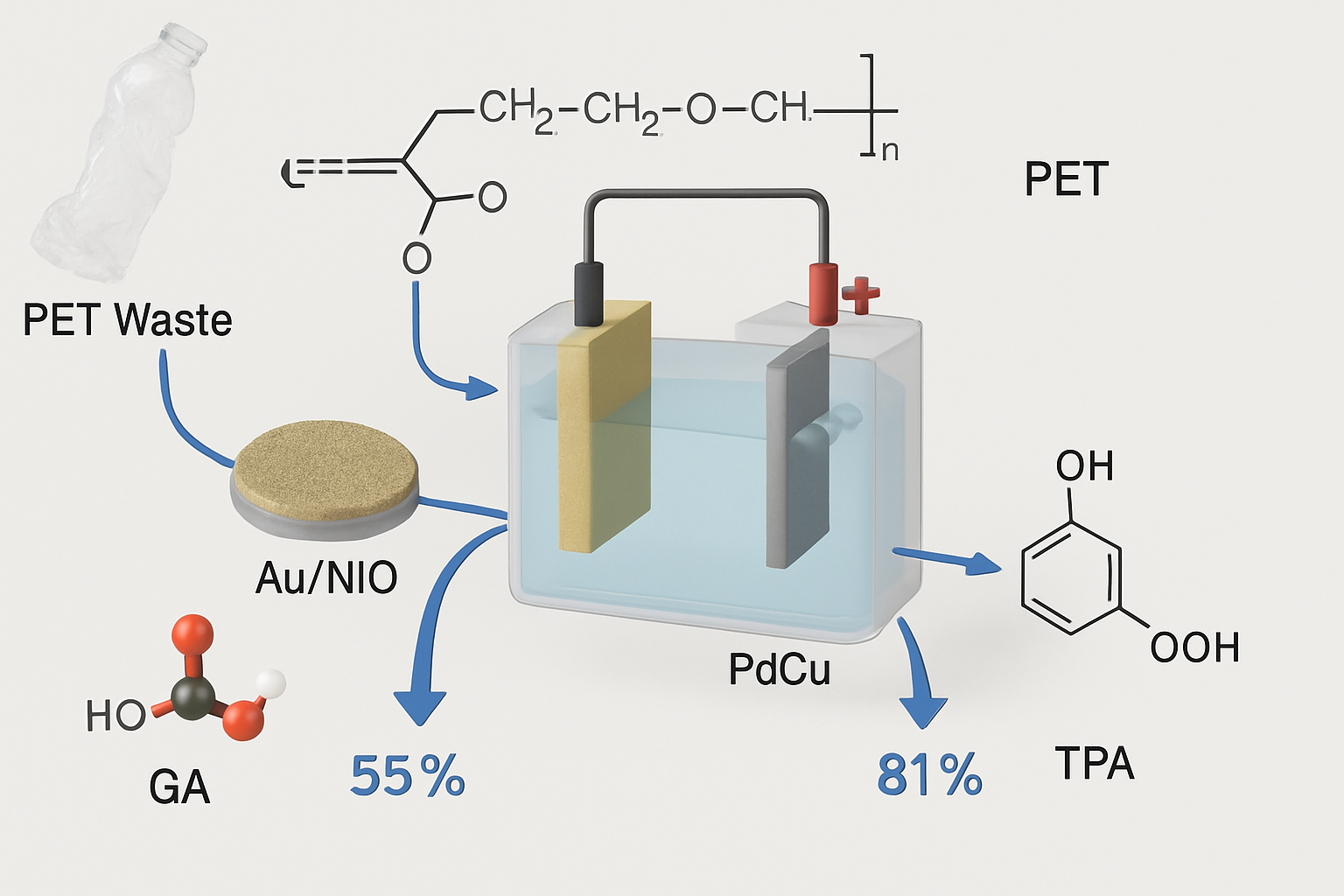
The choice of catalyst material and the design of the electrocatalytic system are critical for achieving high efficiency and selectivity. For example, Chen et al. (2024) demonstrated a one-step tandem technique using an Au/NiO catalyst with abundant oxygen vacancies to enable thermal catalytic oxidation upcycling of PET to TPA and GA, with 99% TPA yield and 87.6% GA yield. The catalyst's oxygen vacancies were discovered to accelerate PET hydrolysis and promote EG adsorption and oxidation. Furthermore, the development of unique reactor designs and separation methods, such as the green separation method for high-purity GA developed by Wang et al. (2025), is critical for the scalable and cost-effective upcycling of PET waste.
Expanding Electrocatalysis to Other Plastic Wastes
While PET upcycling has received a lot of interest, electrocatalytic techniques are also being investigated for other types of plastic trash. Zhang et al. (2025) reported on a flexible organo-photocatalytic upcycling system that uses a phenothiazine derivative to selectively deconstruct a variety of commodity polymers, including polyethylene (PE), polypropylene (PP), polystyrene (PS), and polyvinyl chloride (PVC), into useful small molecules. Although not entirely electrocatalytic, this study emphasizes the possibility of catalytic methods for degrading various plastic wastes under moderate conditions.
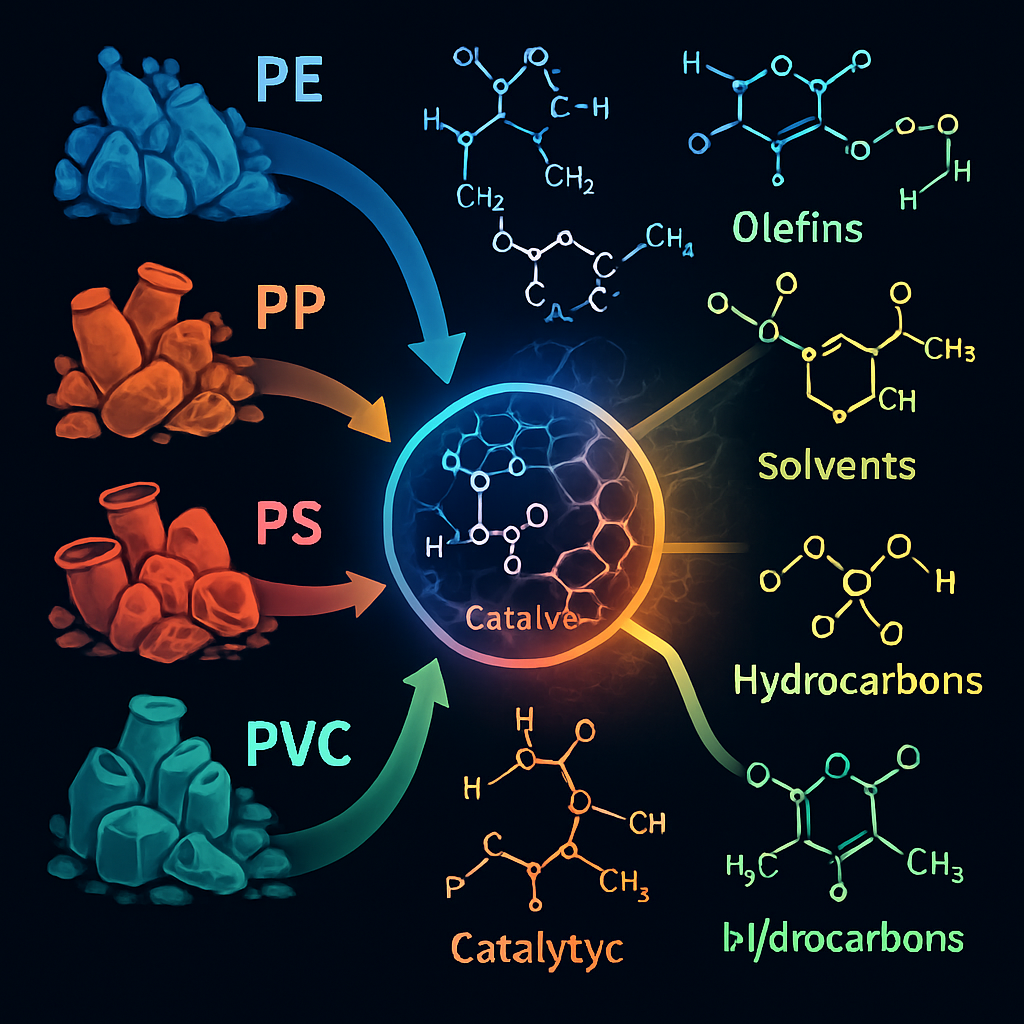
The combination of electrocatalysis with other technologies, such as photocatalysis or bio-catalysis, may provide synergistic benefits for upcycling mixed plastic waste streams. For example, the use of biochar-based catalysts (BBCs) in the catalytic conversion of plastic waste into fuels, as studied by Li et al. (2025), shows the potential of integrating diverse catalytic techniques for plastic valorization. Further research into electrocatalytic systems capable of selectively converting a larger range of plastic wastes, including difficult-to-recycle polymers, is critical for developing comprehensive plastic waste management solutions.
Challenges and Future Perspectives in Electrocatalytic Upcycling
Despite promising breakthroughs, numerous obstacles must be overcome in order to commercialize electrocatalytic plastic upcycling. Key research priorities include improving catalyst stability and lifetime, increasing reaction rates and energy efficiency, and developing cost-effective and scalable reactor designs. Understanding the complex reaction pathways and catalyst deactivation mechanisms is critical for creating strong and efficient electrocatalytic systems. Furthermore, the influence of contaminants and additives commonly seen in post-consumer plastic waste on catalyst performance and product purity must be carefully studied.

Future research should concentrate on developing novel catalyst materials with improved activity and selectivity, such as heteroatom-doped carbons (Kömür et al., 2025) or metal-organic frameworks (MOFs) derived from plastic waste components (Zhou et al., 2024). The use of renewable energy sources to power electrocatalytic processes will increase the sustainability of plastic upcycling. Furthermore, performing thorough life cycle assessments (LCAs) and techno-economic analyses (TEAs), as demonstrated by Cao et al. (2024) for PET alcoholysis, is critical for evaluating the environmental and economic viability of electrocatalytic upcycling technologies and guiding their development toward industrial application. Integrating these technologies into a larger circular economy framework necessitates collaboration between academics, industry, and policymakers to establish enabling legislation and infrastructure.
Conclusion
Electrocatalytic upcycling of plastic waste into high-value monomers provides a viable path to a circular plastics economy. This technology not only tackles the environmental concerns connected with plastic pollution, but it also creates new options for producing valuable chemicals from waste resources. Recent breakthroughs in catalyst creation, reactor design, and process optimization have demonstrated the enormous potential of electrocatalysis for selectively and efficiently converting plastic waste. However, in order to achieve widespread industrial adoption, more research is needed to address current difficulties such as catalyst stability, reaction rates, and scalability.
Future efforts should be focused on developing robust and cost-effective electrocatalytic systems capable of handling diverse and contaminated plastic waste streams. Integrating electrocatalysis with other innovative recycling technologies and utilizing renewable energy sources will improve the sustainability and economic feasibility of plastic upcycling. By embracing a holistic approach that includes technological innovation, policy support, and stakeholder collaboration, we can accelerate the transition to a circular economy in which plastic waste is viewed as a valuable resource rather than a burden, contributing to a more sustainable and resource-efficient future.
References
- Cao, J. et al. (2024). Depolymerization mechanisms and closed-loop assessment in polyester waste recycling. Nature Communications. https://doi.org/10.1038/s41467-024-50702-5
- Chen, Q. et al. (2024). Catalytic oxidation upcycling of polyethylene terephthalate to commodity carboxylic acids. Nature Communications. https://doi.org/10.1038/s41467-024-54822-w
- Han, S. et al. (2025). Pulsed electrosynthesis of glycolic acid through polyethylene terephthalate upcycling over a mesoporous PdCu catalyst. Nature Communications. https://doi.org/10.1038/s41467-025-58813-3
- Kömür, A. I. et al. (2025). Nature’s blueprint for energy: biomass-derived heteroatom-doped graphene materials for advanced energy applications. Carbon Letters. https://doi.org/10.1007/s42823-025-00892-9
- Li, F. et al. (2025). Biochar-based catalytic upgrading of plastic waste into liquid fuels towards sustainability. Communications Earth & Environment. https://doi.org/10.1038/s43247-025-02286-1
- Wang, Y. et al. (2025). Scale-up upcycling of waste polyethylene terephthalate plastics to biodegradable polyglycolic acid plastics. Nature Communications. https://doi.org/10.1038/s41467-025-59667-5
- Zhang, S. et al. (2025). Facile visible-light upcycling of diverse waste plastics using a single organocatalyst with minimal loadings. Nature Communications. https://doi.org/10.1038/s41467-025-59540-5
- Zhou, F. et al. (2024). Sustainable conversion of polyethylene plastic bottles into terephthalic acid, synthesis of coated MIL-101 metal–organic framework and catalytic degradation of pollutant dyes. Scientific Reports. https://doi.org/10.1038/s41598-024-60363-5
- Roychand, R. et al. (2024). A Comprehensive Review on the Thermochemical Treatment of Plastic Waste to Produce High Value Products for Different Applications. Materials Circular Economy. https://doi.org/10.1007/s42824-024-00157-2
- Osman, A. I. et al. (2024). Synergistic technologies for a circular economy: upcycling waste plastics and biomass. Frontiers of Chemical Science and Engineering. https://doi.org/10.1007/s11705-024-2507-0
- Zhang, X. et al. (2024). Crucial role of pre-treatment in plastic photoreforming for precision upcycling. npj Materials Sustainability. https://doi.org/10.1038/s44296-024-00045-5
- Alaraby, M. et al. (2025). Occurrence, analysis, and toxicity of polyethylene terephthalate microplastics: a review. Environmental Chemistry Letters. https://doi.org/10.1007/s10311-025-01841-8
- Mohamed, R. Y. A. et al. (2025). Plasma catalysis for sustainable industry: lab-scale studies and pathways to upscaling. Discover Applied Sciences. https://doi.org/10.1007/s42452-025-06718-7
- Amparán, M. A. A. et al. (2025). Review and future outlook for the removal of microplastics by physical, biological and chemical methods in water bodies and wastewaters. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-025-13883-0
- Silva, W. C. H. et al. (2024). Sustainable Synthesis of Graphene Oxide from Waste Sources: A Comprehensive Review of Methods and Applications. Materials Circular Economy. https://doi.org/10.1007/s42824-024-00117-w
- Varadharajan, S. et al. (2024). Green synthesis and multifaceted applications: challenges and innovations in carbon dot nanocomposites. Discover Nano. https://doi.org/10.1186/s11671-024-04124-3
- Farghali, M. et al. (2024). Strategies for ammonia recovery from wastewater: a review. Environmental Chemistry Letters. https://doi.org/10.1007/s10311-024-01768-6
- Kang, X. et al. (2024). Tungsten pnictides for water electrolysis: advances and perspectives. Tungsten. https://doi.org/10.1007/s42864-024-00268-y
- Gao, X. et al. (2024). Next-Generation Green Hydrogen: Progress and Perspective from Electricity, Catalyst to Electrolyte in Electrocatalytic Water Splitting. Nano-Micro Letters. https://doi.org/10.1007/s40820-024-01424-2
- Catizane, C. et al. (2025). Mechanisms of electrochemical hydrogenation of aromatic compound mixtures over a bimetallic PtRu catalyst. Communications Chemistry. https://doi.org/10.1038/s42004-025-01413-5