Echocardiography-Guided Cryoablation: Using Real-Time Ultrasound Imaging to Control Ice Ball Formation and Minimize Collateral Tissue Damage During Cardiac Arrhythmia Treatment

Cryoballoon ablation has become a cornerstone therapy for atrial fibrillation (AF), offering a durable and efficient method for electrically isolating the pulmonary veins (PVs), the primary source of aberrant signals in many patients. The procedure's success hinges on creating transmural, lasting lesions by applying freezing temperatures to the cardiac tissue. However, this process is largely executed 'blind,' with operators relying on indirect surrogates like balloon temperature, time, and fluoroscopic positioning to infer the effectiveness and safety of the energy application. This lack of direct visual feedback creates a fundamental challenge: the risk of incomplete lesion formation, leading to arrhythmia recurrence, is balanced against the risk of over-application, which can cause severe damage to adjacent structures like the phrenic nerve and esophagus.
This article proposes a paradigm shift from this surrogate-based approach to a visually-directed one, leveraging real-time intracardiac echocardiography (ICE) as the primary guidance system. While ICE is already used to reduce radiation exposure and guide catheter navigation, its full potential remains untapped. We assert that by using ICE to directly visualize the "ice ball"—the propagating front of frozen tissue—operators can move beyond inference and begin to actively control lesion formation in real time. This advancement promises to transform cryoablation from a standardized protocol into a precise, patient-specific therapy, simultaneously enhancing safety by allowing proactive avoidance of collateral damage and improving efficacy by ensuring complete, durable tissue ablation.
The Limits of Indirect Guidance in Cryoablation
The current standard of care in cryoballoon ablation, while effective, operates with significant informational gaps. The primary measure of success during the ablation is the temperature measured at the surface of the balloon, which is used as a proxy for the temperature of the tissue itself. However, the actual size, depth, and transmurality of the resulting lesion are influenced by a host of variables, including tissue thickness, blood flow, and the quality of contact between the balloon and the vein ostium. An operator cannot see the lesion being created; they can only follow a pre-determined protocol of time and temperature and hope the result is effective.
This lack of vision creates significant safety risks. Phrenic nerve injury (PNI) is the most common complication of cryoablation, occurring when the freezing process damages the nearby right phrenic nerve. The standard prevention technique involves pacing the nerve and ceasing ablation if a loss of capture is detected—a reactive strategy that confirms injury is already occurring (Phkhaladze et al., 2024). Similarly, the esophagus, which runs behind the posterior wall of the left atrium, is susceptible to thermal injury, leading to the rare but often fatal complication of atrio-esophageal fistula. While methods like pressure-based sensors can confirm vein occlusion without radiation-heavy contrast injections (Bengel et al., 2025), and fluoroscopy can show the catheter's position, neither can visualize the crucial interaction between the ice and the delicate, non-target tissues.
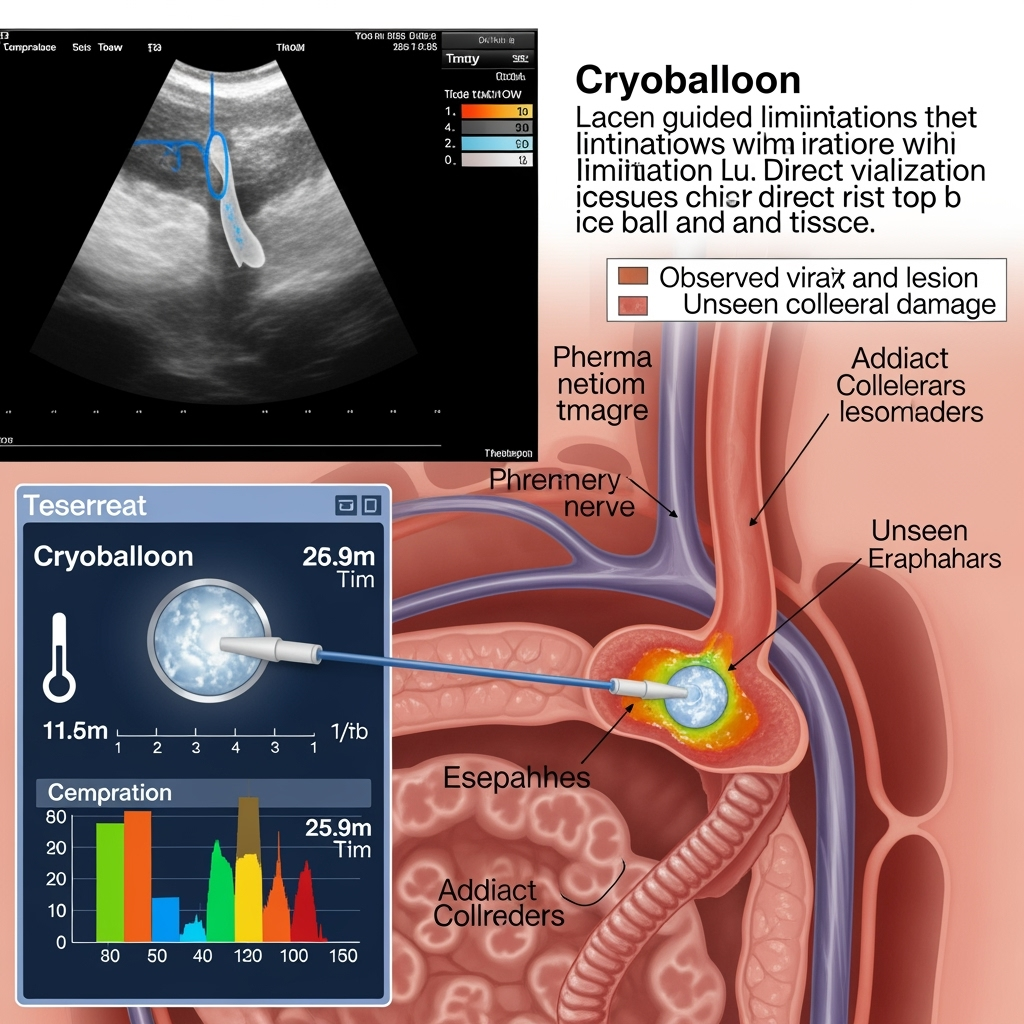
From Navigation to Active Guidance: The Established Role of ICE
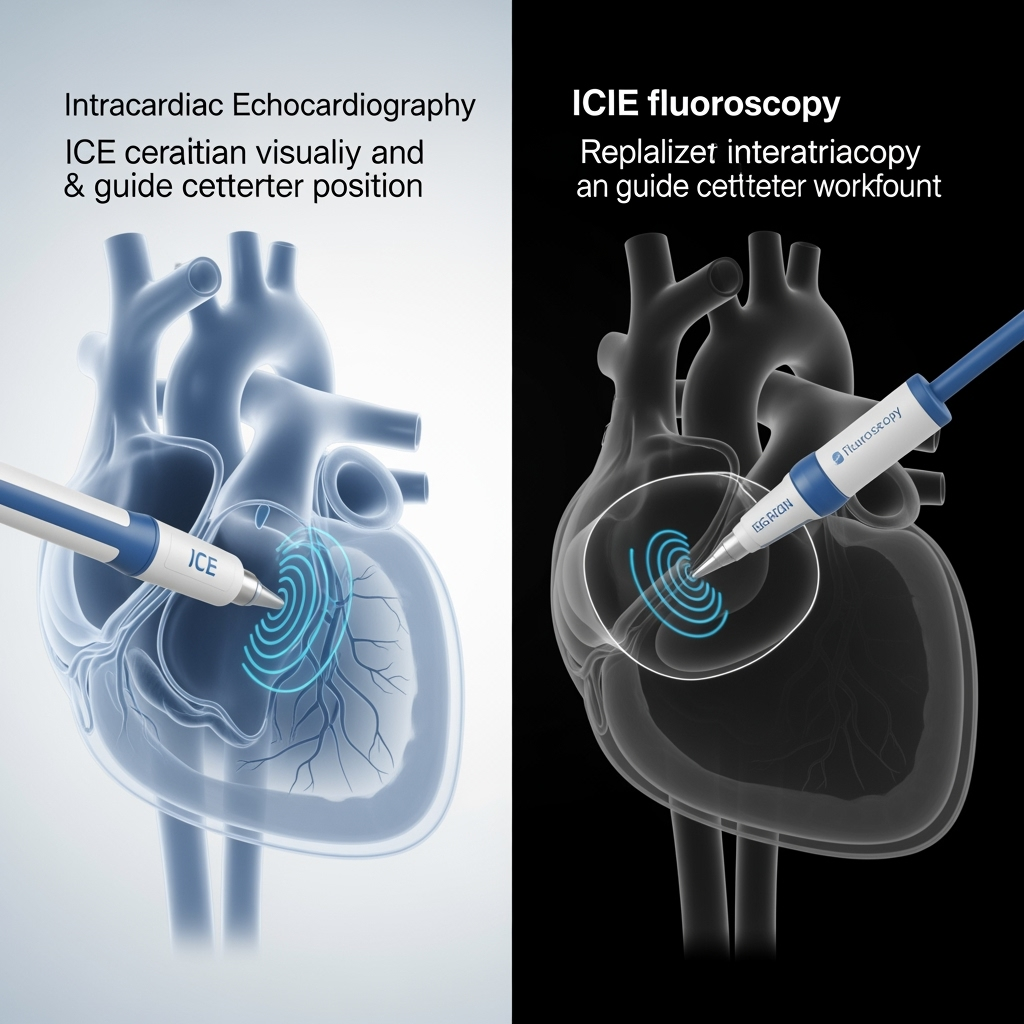
The use of intracardiac echocardiography in electrophysiology is not new. Its ability to provide detailed, real-time images of cardiac structures has already been shown to be a powerful tool for improving procedural safety and efficiency. Studies have definitively demonstrated that ICE guidance significantly reduces fluoroscopy time, radiation dose, and contrast agent volume when compared to traditional fluoroscopy-guided procedures (Tong et al., 2024). This has enabled the rise of "zero-fluoro" approaches, which eliminate radiation exposure for both the patient and the operator, a major advance in occupational and patient safety (Velagic et al., 2025; Miyoshi et al., 2025).
In the context of ablation, ICE provides clear visualization of the interatrial septum for safer transseptal puncture, helps confirm stable catheter-tissue contact, and allows for the precise positioning of catheters within the complex geometry of the left atrium. It effectively replaces the shadows of fluoroscopy with detailed anatomical images. However, to date, its role has largely been confined to navigation and positioning before the application of energy. The next frontier is to use this powerful imaging modality to guide the therapeutic intervention itself.
The Speculative Frontier: Real-Time Ice Ball Visualization and Control
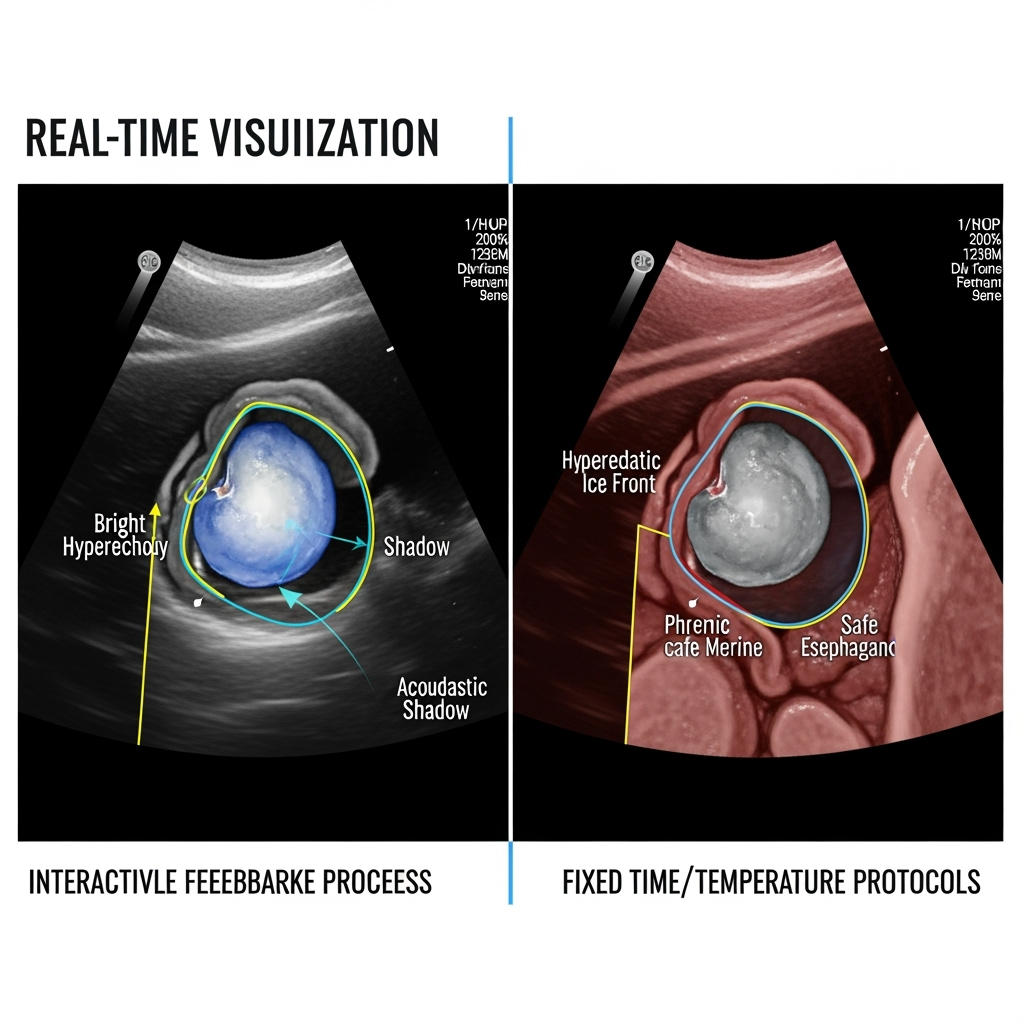
The core of this new paradigm lies in a simple physical principle: frozen tissue is acoustically distinct from unfrozen tissue. The advancing ice ball should therefore be visible on ICE as a hyperechoic (bright) leading edge that casts an acoustic shadow. This real-time visual feedback of the lesion itself opens the door to a more controlled, intelligent, and personalized ablation strategy. Instead of adhering to a rigid protocol (e.g., a 180-second freeze), the operator can titrate the cryoablation dose dynamically, continuing the freeze until the visualized ice ball achieves a target dimension known to be transmural and durable, while ensuring it remains a safe distance from critical structures.
This approach transforms safety from a reactive to a proactive strategy. An operator could directly see the ice ball expanding toward the pericardial space where the phrenic nerve lies. The ablation could be stopped the moment the ice front reaches a predetermined safety margin, before any functional nerve impairment occurs. This proactive, visual-based safety protocol is a significant conceptual advance over the current standard of ceasing ablation only after nerve dysfunction has begun (Phkhaladze et al., 2024). Likewise, the esophagus could be directly imaged, and the ablation modulated or terminated if the ice ball is seen encroaching upon its wall. This method provides a level of anatomical certainty that is impossible with fluoroscopy or esophageal temperature probes alone.
Conclusion
Echocardiography-guided cryoablation is not merely an incremental improvement; it represents a fundamental shift in procedural philosophy. By enabling direct visualization of the ice ball, ICE can move cryoablation beyond its reliance on imperfect surrogate metrics and into an era of direct, real-time therapeutic control. This approach offers the potential to create more durable lesions tailored to individual patient anatomy, which may improve long-term freedom from arrhythmia. More importantly, it provides a proactive mechanism for avoiding collateral damage, a critical step in making a safe procedure even safer. While novel energy sources like pulsed-field ablation (PFA) garner attention for their inherent tissue-selectivity (Yogarajah et al., 2025), optimizing established therapies like cryoablation through advanced imaging presents a parallel and powerful pathway for innovation. Further research should focus on developing standardized protocols, automated imaging software for ice ball detection, and randomized controlled trials to validate whether this visually-guided strategy demonstrably improves both the efficacy and safety of cardiac cryoablation.
References
- Bengel, P. et al. (2025). Comparison between contrast-guided and pressure-guided ablation using the novel pressure visualization tool for cryoballoon pulmonary vein isolation. Heart and Vessels. https://doi.org/10.1007/s00380-025-02574-y
- Miyoshi, M. et al. (2025). Radiation safety in catheter ablation: clinical value of real-time operator dosimetry. Heart and Vessels. https://doi.org/10.1007/s00380-025-02567-x
- Phkhaladze, K. et al. (2024). A new stepwise approach to minimize phrenic nerve injury during cryoballoon pulmonary vein isolation. Journal of Interventional Cardiac Electrophysiology. https://doi.org/10.1007/s10840-024-01953-1
- Tong, Y. et al. (2024). Real-world evaluation of intracardiac echocardiography guided radio-frequency catheter ablation for atrial fibrillation: a retrospective cohort study. Scientific Reports. https://doi.org/10.1038/s41598-024-83186-w
- Velagic, V. et al. (2025). Feasibility and safety of zero-fluoro, “apron-less” approach to repeat pulmonary vein isolation procedures using radiofrequency energy after initial cryoballoon ablation. Scientific Reports. https://doi.org/10.1038/s41598-025-87940-6
- Yogarajah, J. et al. (2025). Acute outcomes of cryoballoon vs. circular vs. pentaspline pulsed-field ablation catheters in combined pulmonary vein isolation and roof line ablation. Journal of Interventional Cardiac Electrophysiology. https://doi.org/10.1007/s10840-025-02078-9