Magnetoencephalography with Diamond Nitrogen-Vacancy Centers: Using Quantum Defects to Non-Invasively Map Neural Oscillations at Unprecedented Spatiotemporal Resolution
Magnetoencephalography (MEG) stands as a powerful, non-invasive neuroimaging technique that directly measures the faint magnetic fields generated by synchronous neural activity. It offers superior temporal resolution to functional magnetic resonance imaging (fMRI), tracking brain processes on a millisecond scale. However, conventional MEG technology is fundamentally constrained by its reliance on superconducting quantum interference devices (SQUIDs), which must be cryogenically cooled to near absolute zero. This requirement necessitates bulky, thermally-insulated helmets that create a significant standoff distance from the scalp, inherently limiting spatial resolution and creating an expensive, inflexible, and cumbersome imaging environment. A new frontier in quantum sensing, based on nitrogen-vacancy (NV) centers in diamond, promises to shatter these limitations. These atom-sized quantum defects operate as highly sensitive magnetometers at room temperature, opening the door to a new generation of wearable, high-resolution MEG systems that could fundamentally transform our ability to map the human brain's functional architecture. This article will explore the physical principles of NV center magnetometry, review its demonstrated applications in biosensing, and propose a speculative yet grounded vision for a next-generation "quantum neuro-imager" capable of mapping not just magnetic field strength, but the full magnetic field vector, to resolve neural dynamics at the level of cortical columns.
The State of the Art: From Cryogenic Giants to Wearable Sensors
Traditional MEG systems detect the magnetic fields produced by the summed postsynaptic potentials of thousands of simultaneously active pyramidal neurons. The current gold standard, SQUID-based MEG, has provided invaluable insights into neural oscillations, cognitive processes, and the localization of epileptic foci. However, the physics of magnetic fields dictates that their strength decays rapidly with distance. The necessary gap between the cryogenically cooled SQUIDs and the subject's head blurs the magnetic signal, making it challenging to precisely localize its source—a limitation known as the ill-posed inverse problem. In recent years, optically pumped magnetometers (OPMs) have emerged as a wearable alternative that can be placed directly on the scalp, eliminating the need for cryogenics and improving spatial resolution. While a major advance, the pursuit of ever-higher sensitivity and denser sensor arrays continues.
Diamond NV centers represent a quantum leap in this progression. An NV center is a point defect in the diamond lattice where a nitrogen atom substitutes a carbon atom adjacent to a lattice vacancy. This defect possesses a quantum spin state that is highly sensitive to local magnetic fields. By initializing the spin with a green laser and reading out its state via red fluorescence, changes in the magnetic field can be measured with remarkable precision. The key advantages of NV centers are profound: they operate under ambient conditions, are inherently biocompatible and robust, and their atomic size allows for the theoretical possibility of creating dense sensor arrays with a spatial resolution far exceeding any current technology.
Experiments have already confirmed the potential of NV-based sensors, demonstrating the detection of magnetic fields from isolated neurons (Hansen et al., 2023), living muscle tissue (Webb et al., 2021), and even the hearts of small animals (Arai et al., 2022), proving their sensitivity is commensurate with biological signals.

Re-engineering MEG with Scalp-Mounted NV Arrays
The most direct application of this technology involves replacing the SQUID or OPM sensors with a flexible, high-density array of diamond NV magnetometers integrated into a wearable cap. Placing sensors microns to millimeters from the scalp would drastically enhance the signal-to-noise ratio and provide a far more detailed map of the magnetic field topography. This would allow researchers to move beyond the coarse-grained images of today and begin to resolve the activity of smaller, more localized neural ensembles, such as functional cortical columns. Simulations have suggested that with sufficient sensor density and sensitivity, it may be possible to achieve single-neuron-resolved 3D reconstruction of activity (Parashar et al., 2020) and image the dynamics of neural networks within brain slices (Karadas et al., 2018).
This vision is not without significant engineering challenges. Achieving the femtotesla-per-root-hertz (fT/√Hz) sensitivity required to detect the subtlest neural signals remains a primary goal. Furthermore, fabricating large-scale, flexible arrays where each NV center has uniform properties, such as a long and homogeneous spin-dephasing time, is a critical hurdle being actively addressed (Shinei et al., 2025). Photonic structures, such as diamond micro-resonators, are being developed to enhance light collection efficiency and boost the sensitivity of on-chip devices (Katsumi et al., 2025), paving the way for scalable production.

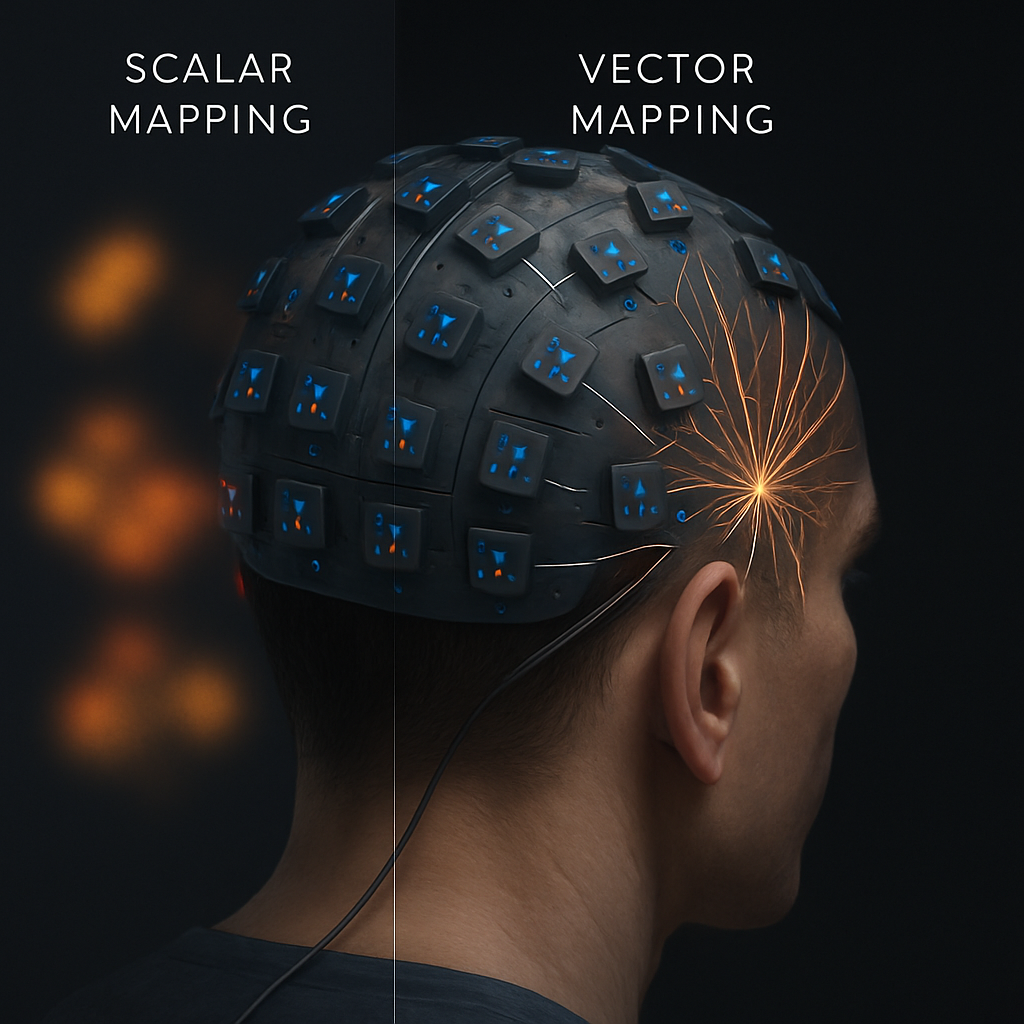
Speculative Leap: From Scalar Maps to Vector-Field Neuro-imaging
Beyond simply improving existing MEG, NV centers offer the potential for a qualitatively new kind of neuro-imaging. A critical, often overlooked limitation of current MEG is that it primarily measures the component of the magnetic field that is perpendicular to the sensor array. This provides an incomplete picture. The unique, diamond-lattice-defined spin axes of NV centers allow them to be configured as full vector magnetometers, capable of measuring all three spatial components (x, y, z) of the magnetic field simultaneously (M. Gilardoni et al., 2025).
We propose a future where NV-MEG moves beyond scalar mapping to vector-field neuro-imaging. An array of such sensors would not just show "hotspots" of activity, but would map the full magnetic field vector at thousands of points across the scalp. This incredibly rich dataset would provide unprecedented constraints for solving the inverse problem. By knowing the direction and magnitude of the field at each point, source localization algorithms could distinguish between different source configurations (e.g., radial vs. tangential dipoles, or simple vs. complex source geometries) with an accuracy that is currently unimaginable. This could allow researchers to non-invasively differentiate the activity of neurons in different cortical layers or distinguish the signals from adjacent but functionally distinct neuronal populations. This would constitute a paradigm shift in non-invasive electrophysiology, transforming MEG from a blurry imaging tool into a precise instrument for dissecting neural circuitry.
Conclusion
The integration of diamond NV centers into magnetoencephalography promises to overcome the most significant limitations of current technology, paving the way for a new generation of high-resolution, room-temperature, and wearable brain imagers. The primary challenges are now centered on materials science and quantum engineering: pushing sensor sensitivity into the femtotesla range and scaling up the fabrication of uniform, dense sensor arrays on flexible substrates. While these are non-trivial obstacles, the progress is rapid and the motivation is immense. The speculative vision of a "quantum neuro-imager" that captures the full magnetic vector field represents a grand challenge for the field, but one with a transformative payoff. Success would provide an unprecedented, non-invasive window into the live, high-speed computations of the human brain, with profound implications for basic neuroscience, clinical diagnostics, and the development of next-generation brain-computer interfaces.
References
- Arai, K. et al. (2022). Millimetre-scale magnetocardiography of living rats with thoracotomy. Communications Physics. https://doi.org/10.1038/s42005-022-00978-0
- Ferrante, O. et al. (2025). Adversarial testing of global neuronal workspace and integrated information theories of consciousness. Nature. https://doi.org/10.1038/s41586-025-08888-1
- García-Colomo, A. et al. (2025). Plasma p-tau231 and NfL differently associate with functional connectivity patterns in cognitively unimpaired individuals. GeroScience. https://doi.org/10.1007/s11357-025-01743-1
- Gilardoni, M. C. et al. (2025). A single spin in hexagonal boron nitride for vectorial quantum magnetometry. Nature Communications. https://doi.org/10.1038/s41467-025-59642-0
- Hansen, N. W. et al. (2023). Microscopic-scale magnetic recording of brain neuronal electrical activity using a diamond quantum sensor. Scientific Reports. https://doi.org/10.1038/s41598-023-39539-y
- Karadas, M. et al. (2018). Feasibility and resolution limits of opto-magnetic imaging of neural network activity in brain slices using color centers in diamond. Scientific Reports. https://doi.org/10.1038/s41598-018-22793-w
- Katsumi, R. et al. (2025). High-sensitivity nanoscale quantum sensors based on a diamond micro-resonator. Communications Materials. https://doi.org/10.1038/s43246-025-00770-x
- Parashar, M., Saha, K., & Bandyopadhyay, S. (2020). Axon hillock currents enable single-neuron-resolved 3D reconstruction using diamond nitrogen-vacancy magnetometry. Communications Physics. https://doi.org/10.1038/s42005-020-00439-6
- Shinei, C. et al. (2025). Homogeneous spin-dephasing time of NV− centre in millimetre-scale 12C-enriched high-pressure high-temperature diamond crystals. Communications Materials. https://doi.org/10.1038/s43246-025-00782-7
- Webb, J. L. et al. (2021). Detection of biological signals from a live mammalian muscle using an early stage diamond quantum sensor. Scientific Reports. https://doi.org/10.1038/s41598-021-81828-x