Designer Glycans: Engineering Novel Carbohydrate Structures for Precision Therapeutics
Carbohydrates, or glycans, are fundamental to virtually every biological process, mediating cell recognition, signaling, and structural integrity. Despite their ubiquity and importance, the intricate complexity of glycans has historically posed challenges for their precise manipulation and therapeutic application. Traditional therapeutics often overlook the profound impact of glycosylation, or suffer from heterogeneous glycoforms that can affect efficacy and safety.
However, a new era is dawning where advances in synthetic glycobiology, chemoenzymatic methods, and our understanding of glycan function are enabling the creation of 'designer glycans.' These are novel carbohydrate structures, or precisely engineered glycoforms on existing molecules, designed with specific therapeutic purposes in mind. This article explores the cutting-edge landscape of designer glycans, highlighting how the deliberate engineering of carbohydrate structures is paving the way for a new generation of precision therapeutics capable of tailored drug efficacy, targeted delivery, and sophisticated immunomodulation.
Glycoengineering Proteins for Enhanced Therapeutic Efficacy
One of the most impactful applications of designer glycans lies in optimizing protein-based therapeutics, particularly monoclonal antibodies (mAbs). The glycosylation profile of an mAb's Fc region critically influences its effector functions, such as antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). For instance, removing fucose residues from Fc glycans (afucosylation) significantly enhances ADCC, a strategy now employed in several approved and investigational antibody therapies (Cree, B. A. C. et al., 2025; Thota, V. N. et al., 2025). Thota et al. (2025) describe a gram-scale synthesis of β-L-carbafucose, a metabolic inhibitor, to produce afucosylated antibodies, exemplifying how chemical tools can precisely modulate cellular glycosylation machinery for therapeutic benefit.
Beyond antibodies, glycoengineering is vital for other protein therapeutics like enzyme replacement therapies (ERTs). The success of ERTs often depends on the correct trafficking of the recombinant enzyme to lysosomes, frequently mediated by mannose-6-phosphate (M6P) receptors. Shinoda and Kitakaze et al. (2025) demonstrate the power of transglycosylation using endo-β-N-acetylglucosaminidases (ENGases) to modify recombinant human α-L-iduronidase (hIDUA) with M6P- or sialic acid-containing N-glycans. This targeted glycan remodeling improved clinical signs in a primate model of Mucopolysaccharidosis type I.
This approach signifies a shift from relying on host cell glycosylation to actively designing and attaching optimal glycans for specific biological outcomes, such as improved pharmacokinetics, reduced immunogenicity, or enhanced receptor targeting. The speculative horizon includes programming multiple glycan-defined attributes onto a single therapeutic protein, creating multifunctional biologics with unprecedented precision.

De Novo Design and Synthesis of Therapeutic Glycans
The field is rapidly moving beyond modifying existing biomolecules to designing and synthesizing glycans de novo as active pharmaceutical ingredients. These synthetic carbohydrate structures can be engineered to mimic, inhibit, or otherwise modulate biological pathways with high specificity. Chen et al. (2025) showcase this by synthesizing a diverse library of fucoidans with defined sulfation patterns, leading to the identification of potent anticoagulants that selectively inhibit the intrinsic coagulation pathway. This rational design approach, based on understanding structure-activity relationships, allows for the creation of glycan drugs with potentially safer profiles than naturally derived heterogeneous polysaccharides. Similarly, Fu et al. (2025) provide structural insights into the activation of TLR4/MD-2 by synthetic LPS-mimicking glycolipids, crucial for designing novel vaccine adjuvants and immunotherapeutics with species-independent activity.
Novel carbohydrate structures are also emerging as advanced biomaterials. Liu et al. (2025, Nature Communications) developed an 'enzyme-proof' glycan glue, glucomannan octanoate, which resists degradation by ECM-cleaving enzymes and promotes nucleus pulposus regeneration in models of intervertebral disc degeneration. This highlights how rationally designed glycans can interact with and modify biological matrices for therapeutic tissue repair. The ability to produce complex natural N-glycans at a gram scale through innovative chemical methods, as described by Zhang et al. (2025, Communications Chemistry), further democratizes access to these molecules for functional studies and therapeutic development.
A key future direction is the integration of computational design and high-throughput synthesis/screening to rapidly discover novel therapeutic carbohydrates with precisely tailored activities, effectively establishing the glycan itself as a programmable drug.
Designer Glycans for Modulating Cellular Interactions and Pathways in Disease
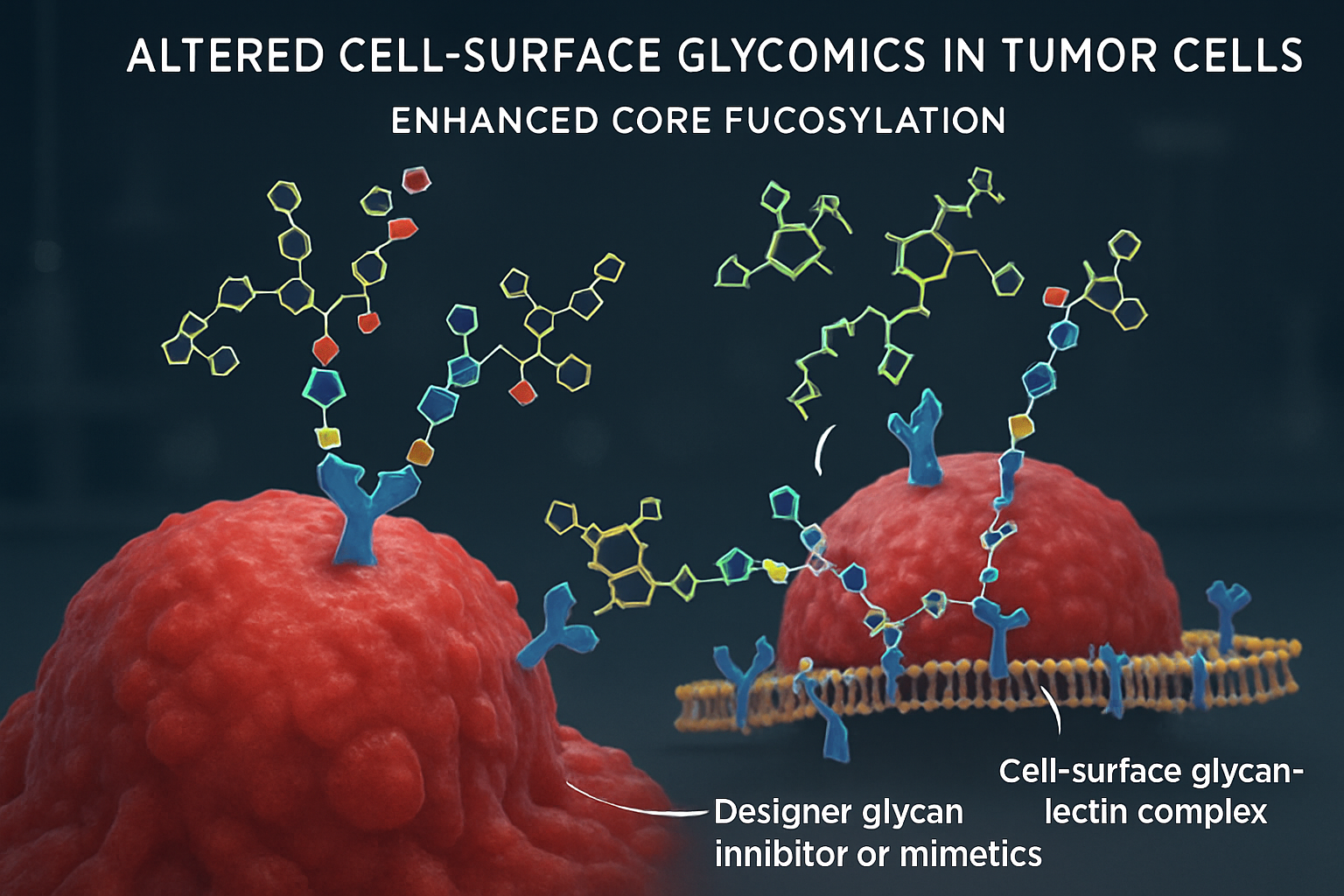
Glycans on the cell surface and in the extracellular matrix form a dense 'glycocalyx' that mediates a vast array of cellular interactions and signaling events crucial in health and disease. Aberrant glycosylation is a hallmark of many diseases, particularly cancer, offering unique targets for designer glycans. Zhu et al. (2025) found that MYCN-amplified neuroblastomas exhibit increased core fucosylation driven by GMDS, identifying a novel metabolic vulnerability that could be targeted by inhibiting de novo GDP-fucose production. This suggests that designer glycans or glycomimetic inhibitors could selectively target cancer-specific glycosylation pathways.
Glycan-lectin interactions are pivotal in immune regulation and cancer progression. Galectins, a family of glycan-binding proteins, are often dysregulated in cancer, contributing to tumor growth, angiogenesis, and immune evasion (Zhang, N. et al., 2025, Biomarker Research; Novák, J. et al., 2025). Kam et al. (2024) showed that cell-associated galectin-9 in nasopharyngeal carcinoma can confer resistance to CTL-mediated killing by inducing autophagy in tumor cells. This opens avenues for designer glycans or glycan-mimetics that block these pathological galectin interactions, thereby restoring anti-tumor immunity.
Furthermore, while direct glycan-based drug delivery systems are still nascent in the abstracts reviewed, concepts like mannosylated albumin for targeted interferon delivery (Fukuda, R. et al., 2025) underscore the potential of using glycans to direct therapeutics to specific cells or tissues. The speculative leap involves creating 'glycan editors' – molecules that can selectively remodel the cell surface glycome in situ, or 'glycan interference' agents that dynamically block pathogenic interactions, offering a new paradigm in modulating cellular behavior for therapeutic benefit.
Conclusion
The precise engineering of carbohydrate structures, or 'designer glycans,' is catalyzing a paradigm shift in therapeutic development. From enhancing the efficacy of protein biologics to the de novo creation of glycan-based drugs and immunomodulators, this field promises to address previously intractable medical challenges. Key implications include the development of more potent and safer antibodies, novel anticoagulants, sophisticated vaccine adjuvants, regenerative biomaterials, and targeted cancer therapies. However, significant challenges remain, including the inherent complexity of glycan synthesis and analysis, predicting the in vivo behavior and immunogenicity of novel glycan structures, and ensuring the scalability and cost-effectiveness of these advanced manufacturing processes.
Future directions will likely involve the increasing use of artificial intelligence and machine learning for predicting glycan structure-function relationships and designing novel therapeutic carbohydrates. The emergence of 'glyco-pharmacology' as a distinct discipline will focus on understanding how these designer glycans interact with biological systems. Furthermore, the potential for personalized glycan therapies, tailored to an individual's specific glycomic profile or disease state, offers an exciting frontier. A provocative question remains: Are we on the verge of being able to fully write and rewrite the 'glyco-code,' thereby programming cellular behavior and therapeutic outcomes with unprecedented precision, and heralding an era of truly 'glycan-defined medicine'?
References
- Cree, B. A. C. et al. (2025). The Evolution of Anti-CD20 Treatment for Multiple Sclerosis: Optimization of Antibody Characteristics and Function. CNS Drugs. http://dx.doi.org/10.1007/s40263-025-01182-8
- Thota, V. N. et al. (2025). A gram-scale synthesis of β-L-carbafucose for engineering antibody glycans. Communications Chemistry. http://dx.doi.org/10.1038/s42004-025-01523-0
- Shinoda, C. & Kitakaze, K. et al. (2025). N-glycan-modified α-L-iduronidase produced by transgenic silkworms ameliorates clinical signs in a Japanese macaque with mucopolysaccharidosis I. Communications Medicine. http://dx.doi.org/10.1038/s43856-025-00841-7
- Chen, S.-C. et al. (2025). Comprehensive synthesis and anticoagulant evaluation of a diverse fucoidan library. Nature Communications. http://dx.doi.org/10.1038/s41467-025-59632-2
- Fu, Y. et al. (2025). Structural insight into TLR4/MD-2 activation by synthetic LPS mimetics with distinct binding modes. Nature Communications. http://dx.doi.org/10.1038/s41467-025-59550-3
- Liu, Y. et al. (2025). An enzyme-proof glycan glue for extracellular matrix to ameliorate intervertebral disc degeneration. Nature Communications. http://dx.doi.org/10.1038/s41467-025-58946-5
- Zhang, Q. et al. (2025). Streamlined gram-scale natural N-glycan production using reversible tagging after oxidative release of natural glycans. Communications Chemistry. http://dx.doi.org/10.1038/s42004-025-01499-x
- Zhu, B. et al. (2025). GDP-mannose 4,6-dehydratase is a key driver of MYCN-amplified neuroblastoma core fucosylation and tumorigenesis. Oncogene. http://dx.doi.org/10.1038/s41388-025-03297-0
- Zhang, N. et al. (2025). Multifaceted roles of Galectins: from carbohydrate binding to targeted cancer therapy. Biomarker Research. http://dx.doi.org/10.1186/s40364-025-00759-1
- Novák, J. et al. (2025). The sweet and the bitter sides of galectin-1 in immunity: its role in immune cell functions, apoptosis, and immunotherapies for cancer with a focus on T cells. Seminars in Immunopathology. http://dx.doi.org/10.1007/s00281-025-01047-8
- Kam, N.-W. et al. (2024). Cell-associated galectin 9 interacts with cytotoxic T cells confers resistance to tumor killing in nasopharyngeal carcinoma through autophagy activation. Cellular & Molecular Immunology. http://dx.doi.org/10.1038/s41423-024-01253-8
- Fukuda, R. et al. (2025). Lymph node macrophage-targeted interferon alpha boosts anticancer immune responses by regulating CD169-positive phenotype of macrophages. Molecular Cancer. http://dx.doi.org/10.1186/s12943-025-02324-8
- Mamun, M. A. A. et al. (2025). Targeted degradation of extracellular proteins: state of the art and diversity of degrader designs. Journal of Hematology & Oncology. http://dx.doi.org/10.1186/s13045-025-01703-4
- Tian, M. et al. (2025). Glycosylation as an intricate post-translational modification process takes part in glycoproteins related immunity. Cell Communication and Signaling. http://dx.doi.org/10.1186/s12964-025-02216-w
- Liu, Y. et al. (2025). Structural insights into terminal arabinosylation of mycobacterial cell wall arabinan. Nature Communications. http://dx.doi.org/10.1038/s41467-025-58196-5