Investigating the Role of Cerebrospinal Fluid Dynamics in Glymphatic Waste Clearance and its Implications for Neurodegenerative Disease
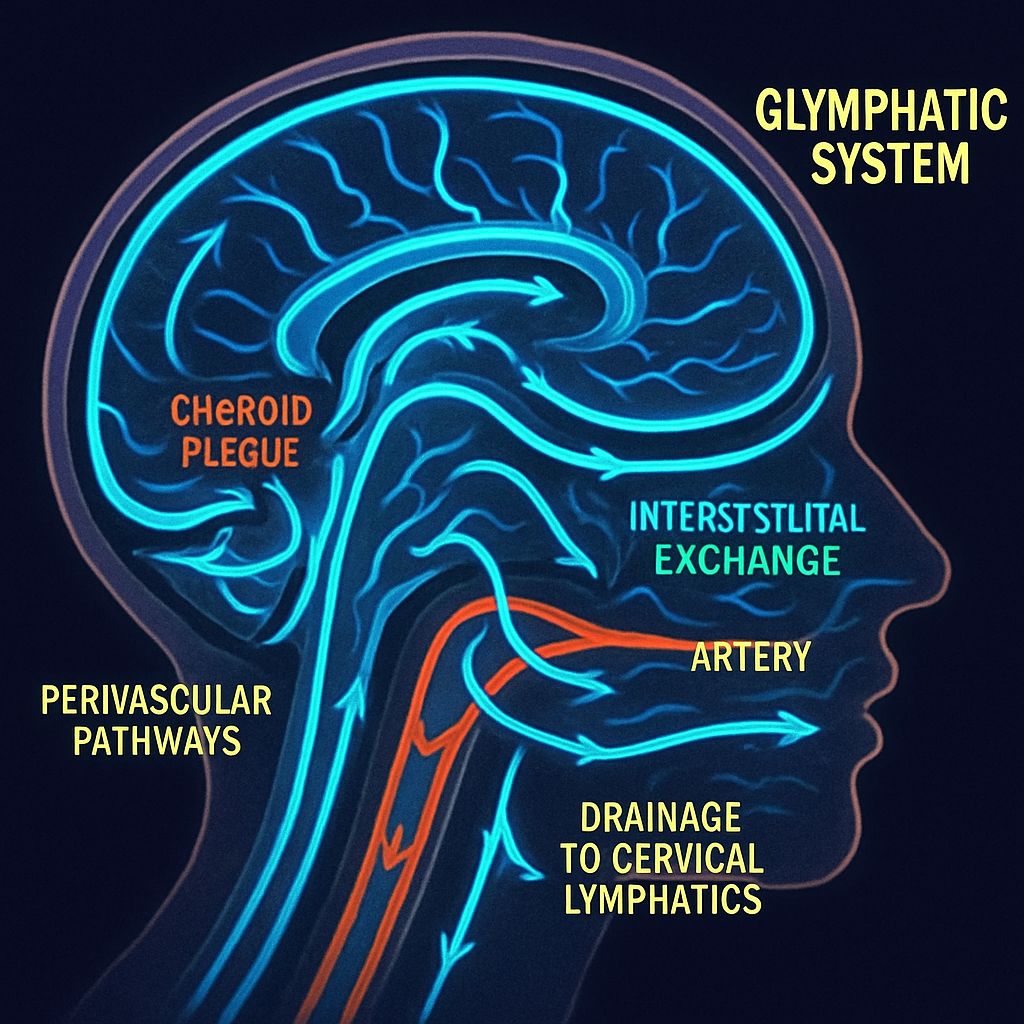
The brain, an organ of immense metabolic activity, has long puzzled scientists with its method of waste disposal. The discovery of the glymphatic system revealed a brain-wide network of perivascular channels that facilitates the clearance of metabolic byproducts, including neurotoxic proteins like amyloid-beta and tau. This system relies on the dynamic flow of cerebrospinal fluid (CSF), which enters the brain parenchyma along arteries, exchanges with the interstitial fluid to collect waste, and is ultimately cleared via drainage pathways including meningeal lymphatic vessels. Emerging evidence suggests that impairment of this intricate fluid transport system is not merely a symptom but a fundamental pathogenic mechanism in a host of neurodegenerative diseases. This article synthesizes recent findings to propose a "systemic plumbing failure" model of neurodegeneration, where breakdowns at distinct checkpoints—CSF production, parenchymal flow, and lymphatic drainage—initiate and accelerate the disease cascade.
The System's Boundaries: Choroid Plexus Inflow and Lymphatic Outflow
The journey of CSF begins at the choroid plexus (CP), the brain's CSF production factory. Pathological changes in this structure may represent the first sign of glymphatic dysfunction. Recent studies have identified CP enlargement as a key imaging biomarker in multiple neurodegenerative disorders. In Progressive Supranuclear Palsy (PSP), increased CP volume correlates directly with the burden of tau pathology in critical subcortical regions (Wang, N. et al., 2025). Similarly, in Parkinson’s disease (PD), CP enlargement is linked to the severity of motor symptoms, an association mediated by glymphatic dysfunction in the basal ganglia (Liu, L. et al., 2025). These findings suggest the "engine" of the glymphatic system may be failing, with the enlarged CP reflecting a dysfunctional, perhaps inflammatory, state that compromises CSF quality and dynamics from the very start.
At the other end of the system lies the final drainage pathway: the dural lymphatic vessels that carry waste-laden CSF to cervical lymph nodes. This "drainage" system is highly vulnerable to age-related decline. Groundbreaking work by Jin et al. (2025) in aged mice revealed a significant reduction in meningeal lymphatics and a corresponding impairment in CSF outflow. Critically, they demonstrated that this deficit is not irreversible. Non-invasive, force-regulated mechanical stimulation of the superficial cervical lymphatics was able to double CSF outflow, effectively correcting the age-related impairment. Further supporting the therapeutic potential of targeting this outflow tract, Keuters et al. (2024) found that inducing the growth of dural lymphatic vessels with vascular endothelial growth factor C (VEGF-C) significantly improved outcomes and enhanced fluid clearance in a mouse model of stroke.

Journey Through the Parenchyma: The Central Role of Astrocytes and AQP4
Between production and drainage, CSF must traverse the dense brain parenchyma. This exchange with interstitial fluid is not a passive process; it is actively facilitated by aquaporin-4 (AQP4) water channels densely expressed on the end-feet of astrocytes, the glial cells that form a critical interface between blood vessels and neurons. Any disruption to this astrocytic-vascular unit can effectively "clog the pipes" within the brain. A study on spontaneously hypertensive rats by Xia et al. (2025) provides a compelling link between vascular disease and glymphatic failure. They found that hypertensive animals exhibited significantly impaired glymphatic clearance, which was associated with reduced AQP4 expression and widespread astrogliosis—a reactive scarring of astrocytes. This suggests that chronic hypertension, a major risk factor for dementia, may exert its neurotoxic effects in part by crippling the AQP4-dependent machinery of glymphatic transport.
The role of astrocytes extends beyond simple fluid transport; they are central regulators of the entire neurovascular unit and sleep-wake cycles (Bellier, F. et al., 2025). Therefore, astrocytic dysfunction represents a critical node linking vascular health, sleep quality, and glymphatic clearance. A failure at this cellular level could decouple the glymphatic system from its primary drivers, leading to a stagnant, toxic microenvironment ripe for the accumulation of pathological proteins.

The Regulatory Network: How Sleep, Nerves, and Rhythms Control the Flow
The glymphatic system is not static; its activity is dynamically regulated, peaking during deep, slow-wave sleep. This crucial link has been elegantly demonstrated in humans for the first time using a novel, non-invasive wireless device that measures brain parenchymal resistance. Dagum et al. (2025) showed that these measurements, which reflect changes in extracellular volume, directly track glymphatic function and confirmed that clearance increases with EEG delta power (a marker of deep sleep) and decreases with factors like elevated heart rate. This provides a direct physiological explanation for the well-established epidemiological link between poor sleep and increased risk for Alzheimer's disease.
This sleep-dependent function is itself likely governed by other brain-wide systems. The circadian system, for instance, imposes a daily rhythm on both CP function and CSF composition (Fame, R.M., 2025), suggesting that a desynchronized internal clock could impair glymphatic readiness. Furthermore, a thought-provoking review by Zhu et al. (2025) highlights the role of the noradrenergic system in modulating glymphatic clearance. This introduces a speculative but powerful hypothesis for a vicious cycle in neurodegeneration: The locus coeruleus, the brain's primary source of norepinephrine, is one of the first sites of tau pathology in Alzheimer's disease. Its early degeneration would compromise noradrenergic signaling, which in turn would impair sleep quality and glymphatic function, thereby accelerating the clearance failure and accumulation of the very proteins that initiated the damage.

Conclusion
The conceptualization of neurodegenerative disorders as a "systemic plumbing failure" of the brain's fluid dynamics shifts the clinical and scientific focus from downstream consequences, like protein plaques, to upstream causes of clearance failure. This opens a new frontier for both diagnostics and therapeutics. We are entering an era where glymphatic function can be non-invasively monitored in humans (Dagum et al., 2025) and where imaging metrics like the DTI-ALPS index (Zhang, S. et al., 2025) and choroid plexus volume (Wang, N. et al., 2025) can serve as actionable biomarkers for disease risk and progression.
This model points toward a multi-pronged therapeutic strategy aimed at restoring hydraulic function across the entire system. Interventions could include non-invasive mechanical stimulation to "unclog the drain" at the cervical lymphatics, therapies aimed at preserving astrocyte health and AQP4 function to "repair the parenchymal pipes," and chronotherapies designed to optimize sleep and circadian rhythms to "restore the flow." By addressing the fundamental fluid dynamics of the brain, we may finally be able to move beyond managing the symptoms of neurodegeneration and towards preventing the catastrophic failure of this most vital of systems.
References
- Bellier, F. et al. (2025). Astrocytes at the heart of sleep: from genes to network dynamics. Cellular and Molecular Life Sciences. https://doi.org/10.1007/s00018-025-05671-3
- Dagum, P. et al. (2025). A wireless device for continuous measurement of brain parenchymal resistance tracks glymphatic function in humans. Nature Biomedical Engineering. https://doi.org/10.1038/s41551-025-01394-9
- Fame, R.M. (2025). Harnessing the circadian nature of the choroid plexus and cerebrospinal fluid. npj Biological Timing and Sleep. https://doi.org/10.1038/s44323-025-00033-5
- Jin, H. et al. (2025). Increased CSF drainage by non-invasive manipulation of cervical lymphatics. Nature. https://doi.org/10.1038/s41586-025-09052-5
- Keuters, M.H. et al. (2024). The Impact of VEGF-C-Induced Dural Lymphatic Vessel Growth on Ischemic Stroke Pathology. Translational Stroke Research. https://doi.org/10.1007/s12975-024-01262-9
- Liu, L. et al. (2025). Choroid plexus enlargement contributes to motor severity via regional glymphatic dysfunction in Parkinson’s disease. npj Parkinson's Disease. https://doi.org/10.1038/s41531-025-00971-8
- Wang, N. et al. (2025). Choroid plexus enlargement correlates with subcortical tau deposition in progressive supranuclear palsy. Fluids and Barriers of the CNS. https://doi.org/10.1186/s12987-025-00663-8
- Xia, Y. et al. (2025). The glymphatic system was impaired in spontaneously hypertensive rats. Scientific Reports. https://doi.org/10.1038/s41598-025-02054-3
- Zhang, S. et al. (2025). Glymphatic dysfunction as a biomarker for post-stroke cognitive impairment. Scientific Reports. https://doi.org/10.1038/s41598-025-04054-9
- Zhu, T.-T. et al. (2025). Noradrenergic modulation of glymphatic clearance: implications for neuropsychiatric disorders and mortality. Molecular Psychiatry. https://doi.org/10.1038/s41380-025-03051-8