Cryo-Volcanic Vent Biofilms on Enceladus: Simulating Chemosynthetic Ecosystems with Soft Robotic Samplers
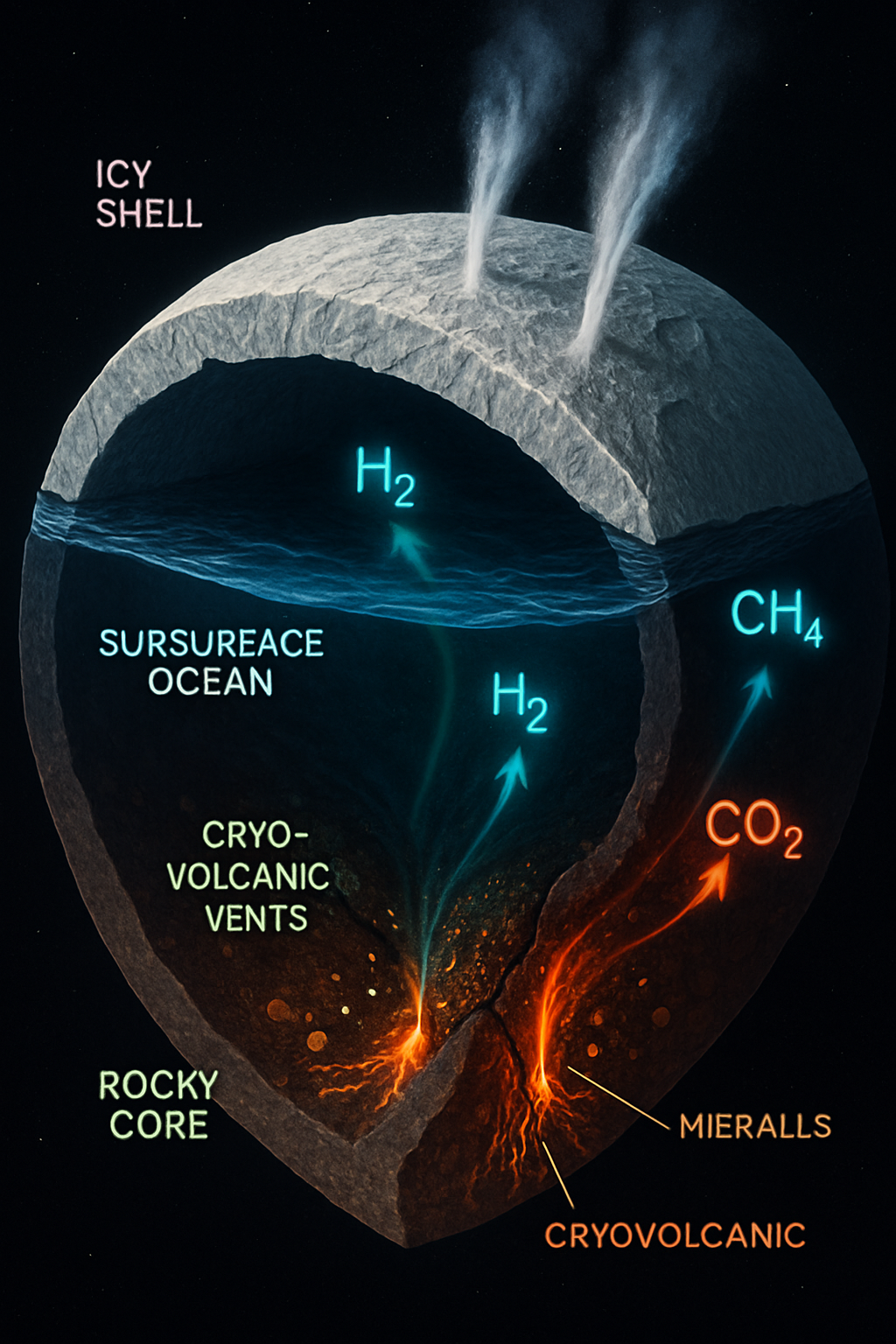
Saturn's moon Enceladus has emerged as a primary target in the search for extraterrestrial life, largely due to the discovery of cryovolcanic plumes erupting from its south polar region, indicative of a subsurface liquid water ocean (Pappalardo, R. T. et al., 2024; Waite, J. H., Jr. et al., 2024). Evidence suggests this ocean is in contact with a rocky core, potentially hosting hydrothermal vent systems analogous to those found in Earth's deep oceans (Schoenfeld, A. M. et al., 2023; Tobie, G. et al., 2025). These vents could provide the chemical energy necessary to support chemosynthetic life, independent of sunlight. While direct evidence of life remains elusive, the conditions raise tantalizing possibilities for microbial ecosystems. This article explores the speculative yet scientifically grounded hypothesis that microbial biofilms—organized communities of microorganisms—could thrive at these Enceladan cryo-volcanic vents. We will delve into their potential characteristics, the challenges and approaches for simulating such ecosystems in laboratory settings, and propose the innovative use of soft robotic samplers as a key technology for their future in-situ detection and investigation.
Enceladus's Cryo-Hydrothermal Systems: A Potential Oasis for Life?
The Cassini mission provided compelling evidence for a global saline ocean beneath Enceladus's icy shell, with ongoing cryovolcanism ejecting ocean material into space (Tobie, G. et al., 2025; Becker, T. M. et al., 2024). Analysis of these plumes revealed water ice, salts, silica nanoparticles (suggesting high-temperature water-rock interactions >90°C), and simple organic molecules, alongside gases like H₂, CH₄, and CO₂ (Hofmann, F. et al., 2025; Waite, J. H., Jr. et al., 2024). The presence of molecular hydrogen is particularly significant, as it is a key energy source for chemosynthetic organisms, similar to those found at terrestrial hydrothermal vents (Keller, L. M. et al., 2025; Colman, D. R. et al., 2024). These conditions strongly point towards active hydrothermal systems at the ocean-core interface, creating chemical gradients that could fuel life. While ocean stratification models suggest that transport from deep vents to the plume source region might be complex and potentially slow (Ames, F. et al., 2025), the very existence of these geochemically rich environments makes them prime locations for habitability. The inferred ocean chemistry, potentially mildly alkaline, further aligns with conditions known to support microbial life in terrestrial extreme environments, such as cold, anoxic, hypersaline springs (Magnuson, E. et al., 2023).

Hypothetical Cryo-Vent Biofilms: Structure, Metabolism, and Biosignatures
On Earth, biofilms are ubiquitous in extreme environments, providing protection, facilitating nutrient acquisition, and supporting complex microbial interactions. At Enceladan cryo-vents, we might hypothesize the existence of analogous structures. These biofilms could be anchored to mineral surfaces near vent orifices, harnessing the chemical energy released. Potential metabolic pathways could include methanogenesis (utilizing H₂ and CO₂), sulfate reduction, and various forms of sulfur cycling, given the likely availability of sulfur compounds from water-rock interactions (Deng, W. et al., 2023; Chen, X. et al., 2023).
The structure of such cryo-biofilms would be adapted to low temperatures (~0°C in the bulk ocean, potentially warmer near vent effluents), high pressures, and the specific geochemistry. They might form filamentous mats or slimy coatings, with extracellular polymeric substances (EPS) uniquely adapted for cryoprotection and adhesion in a saline, dynamic environment. Detecting such biofilms would rely on identifying their biosignatures. Morphological biosignatures could include preserved cellular structures or filamentous textures, which have shown remarkable resilience even under simulated ocean world surface conditions (Vincent, L. N. et al., 2024; Lima-Zaloumis, J. et al., 2022). Chemical biosignatures might encompass specific lipid profiles, pigments (if any non-photosynthetic pigment systems evolve), complex organic molecules within EPS, or characteristic isotopic fractionation patterns. An intriguing, albeit speculative, biosignature could be an "energy-ordered resource stratification" at the micro-scale within the biofilm, reflecting competitive ecosystem dynamics (Goyal, A. & Tikhonov, M., 2025). A suite of multiple biomolecular detections would likely be necessary for robust life detection (Zaman, A. et al., 2024).
Laboratory Simulations: Bridging Theory and Observation
Given the challenges of directly exploring Enceladan vents, laboratory simulations are crucial for testing hypotheses about potential chemosynthetic ecosystems and biofilm formation. High-pressure, low-temperature bioreactors can be designed to mimic the conditions at Enceladan vent interfaces, including temperature gradients, pressure, and the introduction of key chemical substrates (e.g., H₂, CO₂, CH₄, sulfides) identified from plume data and geochemical models (Hofmann, F. et al., 2025). Experiments involving the cultivation of terrestrial extremophiles from analogous environments (e.g., Arctic cold seeps, Antarctic subglacial lakes, deep-sea hydrothermal vents) under these simulated Enceladan conditions could reveal whether they form biofilms, their growth rates, metabolic products, and the composition of their EPS (Magnuson, E. et al., 2023; Hadland, N. et al., 2024).

Furthermore, laboratory studies can investigate the abiotic synthesis of organic molecules under simulated vent conditions (Purvis, G. et al., 2023), providing crucial context for distinguishing potential biosignatures from non-biological chemistry. The stability and alteration pathways of putative biosignatures (both molecular and morphological) under Enceladan conditions (e.g., varying salinity, pH, potential anoxia) also require systematic experimental investigation (Bourmancé, L. et al., 2025; Krýza, O. et al., 2025).
Soft Robotic Samplers: Navigating and Investigating Enceladan Vents
The direct sampling and analysis of material from cryo-volcanic vents or their immediate surroundings on Enceladus present formidable engineering challenges. The environment is remote, cold, potentially characterized by rugged and unknown terrain near vent orifices, and any biological structures like biofilms are likely to be extremely fragile. Traditional rigid robotic samplers, designed for robust geological targets, may be ill-suited for the delicate task of acquiring intact biofilm samples or interacting gently with vent structures.
Herein lies the transformative potential of soft robotics. Inspired by biological organisms, soft robots are constructed from compliant materials, allowing for adaptable morphologies, resilience to damage, and inherently safer, gentler interactions with their environment. For Enceladus vent exploration, soft robotic samplers could offer several unique advantages:
- Gentle Interaction: Tentacle-like manipulators or compliant grippers could delicately detach or scrape biofilm samples from vent surfaces with minimal disturbance, preserving their structural integrity and contextual information.
- Adaptive Locomotion: Soft robotic systems could potentially navigate the complex, uneven, and potentially confined spaces around vent structures more effectively than rigid rovers, perhaps using undulatory or amoeboid forms of movement.
- Conformal Sampling: Soft surfaces could conform to irregular vent textures, maximizing contact for sample acquisition or for deploying in-situ sensors. Suction-based samplers integrated into soft structures could gently gather loose material or plume particles settling near vents.
- Integrated Microfluidics: Soft robotic samplers could incorporate microfluidic channels for immediate sample processing, preservation, or even preliminary analysis, minimizing degradation before return to an orbiter or a lander's main analytical suite.

While the field of soft robotics for space exploration is still nascent (Barnes, J. W. et al., 2011, described an aerial vehicle concept for Titan with different but related challenges of in-situ exploration), its principles are highly relevant to the unique challenges of Enceladan astrobiology. The development of radiation-hardened, cryo-tolerant soft materials and actuators is a key research frontier. Future mission concepts could envision small, autonomous, or tethered soft robotic probes deployed from a lander to specifically target vent sites for biofilm characterization, equipped with miniaturized versions of instruments like those planned for Europa Clipper (e.g., imagers, spectrometers, mass spectrometers) (Blaney, D. L. et al., 2024; Kempf, S. et al., 2025).
Conclusion
The prospect of chemosynthetic biofilms thriving at cryo-volcanic vents on Enceladus offers a compelling, albeit speculative, vision for astrobiology. Such ecosystems would represent life adapting to an environment profoundly different from Earth, yet governed by the universal principles of energy utilization and community organization. Realizing this vision requires an integrative approach: leveraging insights from Earth's extremophile ecosystems, conducting sophisticated laboratory simulations to mimic Enceladan conditions and test hypotheses of biofilm formation and biosignature stability, and pioneering new exploration technologies. Soft robotic samplers, with their potential for gentle and adaptive interaction, represent a particularly promising avenue for future in-situ investigation of these fragile, hypothetical oases. Continued interdisciplinary research, focusing on the development of specific soft robotic prototypes tailored for cryo-environments, refining life detection instrumentation for subtle biofilm signatures, and advocating for dedicated missions to explore Enceladus's ocean floor, will be paramount in our quest to determine if life has indeed taken hold in this distant, icy world. The grand challenge remains to distinguish unequivocally between abiotic organic chemistry and the complex, organized chemical systems indicative of life.
References
- Ames, F., Ferreira, D., Czaja, A., & Masters, A. (2025). Ocean stratification impedes particulate transport to the plumes of Enceladus. Communications Earth & Environment. https://doi.org/10.1038/s43247-025-02036-3
- Barnes, J. W. et al. (2011). AVIATR—Aerial Vehicle for In-situ and Airborne Titan Reconnaissance. Experimental Astronomy. https://doi.org/10.1007/s10686-011-9275-9
- Becker, T. M. et al. (2024). Exploring the Composition of Europa with the Upcoming Europa Clipper Mission. Space Science Reviews. https://doi.org/10.1007/s11214-024-01069-y
- Blaney, D. L. et al. (2024). The Mapping Imaging Spectrometer for Europa (MISE). Space Science Reviews. https://doi.org/10.1007/s11214-024-01097-8
- Bourmancé, L. et al. (2025). The salty tango of brine composition and UV photochemistry effects on Halobacterium salinarum cell envelope biosignature preservation. Communications Biology. https://doi.org/10.1038/s42003-025-08007-w
- Chen, X. et al. (2023). Phylogenetically and metabolically diverse autotrophs in the world’s deepest blue hole. ISME Communications. https://doi.org/10.1038/s43705-023-00327-4
- Colman, D. R. et al. (2024). Covariation of hot spring geochemistry with microbial genomic diversity, function, and evolution. Nature Communications. https://doi.org/10.1038/s41467-024-51841-5
- Deng, W. et al. (2023). Strategies of chemolithoautotrophs adapting to high temperature and extremely acidic conditions in a shallow hydrothermal ecosystem. Microbiome. https://doi.org/10.1186/s40168-023-01712-w
- Goyal, A., & Tikhonov, M. (2025). Energy-ordered resource stratification as an agnostic signature of life. Nature Communications. https://doi.org/10.1038/s41467-025-58206-6
- Hadland, N., Hamilton, C. W., & Duhamel, S. (2024). Young volcanic terrains are windows into early microbial colonization. Communications Earth & Environment. https://doi.org/10.1038/s43247-024-01280-3
- Hofmann, F., Asanova, N., Urso, R. G., & Elsaesser, A. (2025). Exploring the formation and alteration of organics in ice: experimental insights for astrochemistry and space missions. Earth, Planets and Space. https://doi.org/10.1186/s40623-025-02207-8
- Keller, L. M., Colman, D. R., & Boyd, E. S. (2025). Simultaneous aerobic and anaerobic respiration in hot spring chemolithotrophic bacteria. Nature Communications. https://doi.org/10.1038/s41467-025-56418-4
- Kempf, S. et al. (2025). SUDA: A SUrface Dust Analyser for Compositional Mapping of the Galilean Moon Europa. Space Science Reviews. https://doi.org/10.1007/s11214-025-01134-0
- Krýza, O. et al. (2025). Small amounts of dissolved salts increase the mobility of mud flows on Mars and other extraterrestrial bodies. Communications Earth & Environment. https://doi.org/10.1038/s43247-025-02110-w
- Lima-Zaloumis, J. et al. (2022). Microbial biosignature preservation in carbonated serpentine from the Samail Ophiolite, Oman. Communications Earth & Environment. https://doi.org/10.1038/s43247-022-00551-1
- Magnuson, E., Altshuler, I., Freyria, N. J., Leveille, R. J., & Whyte, L. G. (2023). Sulfur-cycling chemolithoautotrophic microbial community dominates a cold, anoxic, hypersaline Arctic spring. Microbiome. https://doi.org/10.1186/s40168-023-01628-5
- Pappalardo, R. T. et al. (2024). Science Overview of the Europa Clipper Mission. Space Science Reviews. https://doi.org/10.1007/s11214-024-01070-5
- Purvis, G. et al. (2023). Generation of long-chain fatty acids by hydrogen-driven bicarbonate reduction in ancient alkaline hydrothermal vents. Communications Earth & Environment. https://doi.org/10.1038/s43247-023-01196-4
- Schoenfeld, A. M., Hawkins, E. K., Soderlund, K. M., Vance, S. D., Leonard, E., & Yin, A. (2023). Particle entrainment and rotating convection in Enceladus’ ocean. Communications Earth & Environment. https://doi.org/10.1038/s43247-023-00674-z
- Tobie, G. et al. (2025). Tidal Deformation and Dissipation Processes in Icy Worlds. Space Science Reviews. https://doi.org/10.1007/s11214-025-01136-y
- Vincent, L. N., Fayolle, E. C., Hodyss, R., Johnson, P. V., & Noell, A. C. (2024). Bacterial spore morphology remains highly recognizable after exposure to simulated Enceladus and Europa surface conditions. Communications Earth & Environment. https://doi.org/10.1038/s43247-024-01872-z
- Waite, J. H., Jr. et al. (2024). MASPEX-Europa: The Europa Clipper Neutral Gas Mass Spectrometer Investigation. Space Science Reviews. https://doi.org/10.1007/s11214-024-01061-6
- Zaman, A. et al. (2024). A multiple biomolecules-based rapid life detection protocol embedded in a rover scientific subsystem for soil sample analysis. Scientific Reports. https://doi.org/10.1038/s41598-024-77808-6