Cryo-Electron Tomography of Intracellular Condensates
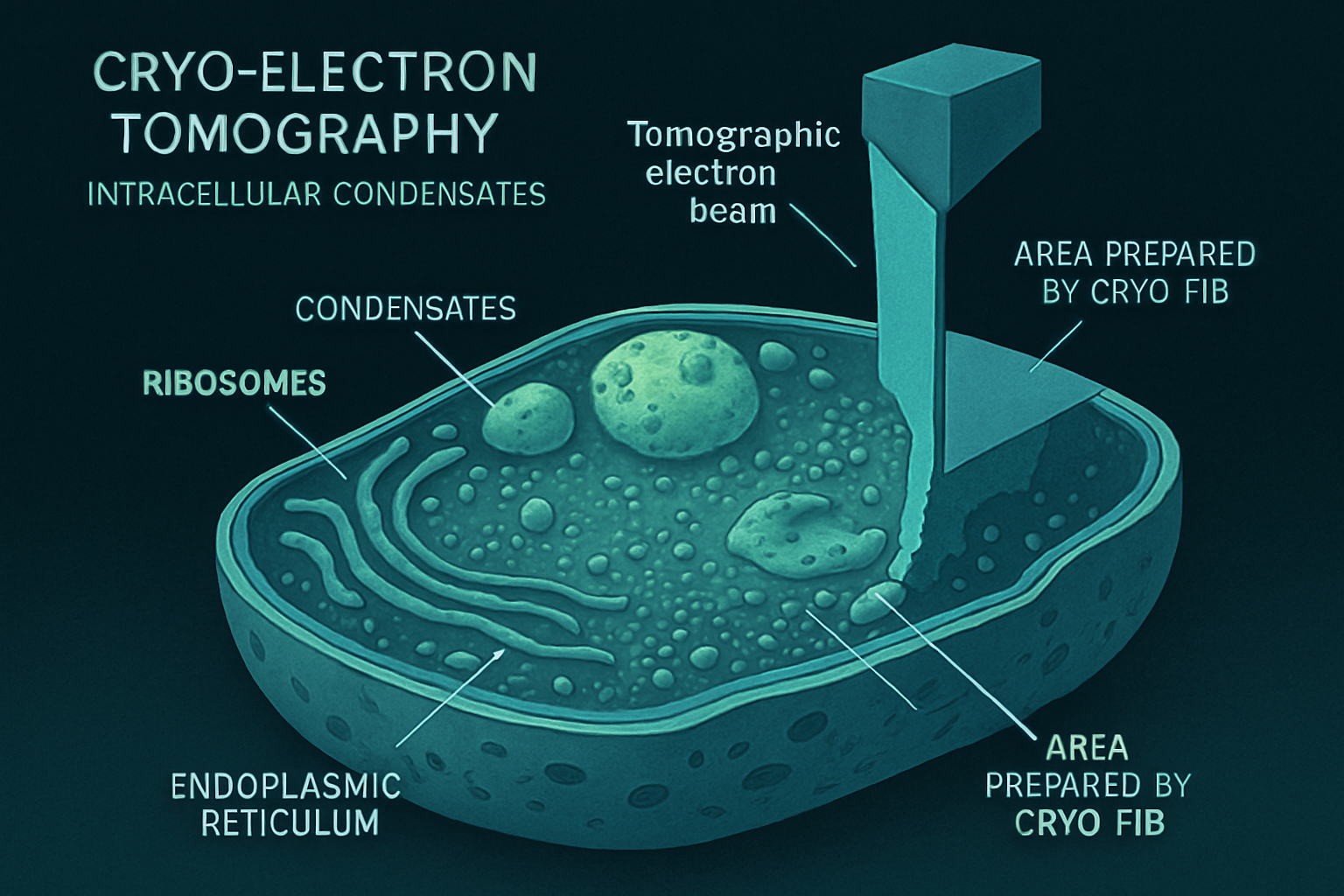
Intracellular condensates, often referred to as membraneless organelles, are dynamic biomolecular assemblies that form through processes like liquid-liquid phase separation (LLPS). These compartments play crucial roles in organizing cellular biochemistry by concentrating specific proteins and nucleic acids, thereby facilitating diverse functions such as RNA metabolism, stress response, signaling, and DNA organization. Understanding their native structure, organization within the crowded cellular environment, and interactions with other organelles is paramount to deciphering their physiological roles and contributions to disease.
Cryo-electron tomography (cryo-ET) has emerged as a revolutionary imaging technique, uniquely capable of visualizing these delicate structures in their near-native, hydrated state within intact cells at macromolecular resolution. This article reviews how cryo-ET is transforming our comprehension of intracellular condensate architecture, the molecular mechanisms governing their formation, their functional implications, and how it fuels novel hypotheses about their orchestration of cellular life. Cryo-ET, particularly when coupled with correlative light and electron microscopy (CLEM) and advanced sample preparation techniques like cryo-focused ion beam (cryo-FIB) milling, allows researchers to pinpoint and dissect condensates within the complex 3D landscape of the cell. This approach bridges the gap between light microscopy observations of condensate dynamics and the high-resolution structural details that underpin their function, providing unprecedented insights into their in situ organization and material properties.
Unveiling Condensate Architecture in Native Cellular Landscapes
A primary strength of cryo-ET is its ability to capture the unadulterated morphology and spatial context of intracellular condensates. Studies using cryo-ET have revealed the intricate architectures of a wide array of these bodies. For instance, viral factories, which are sites of viral replication, have been shown by cryo-ET to be complex condensates recruiting host and viral components, with internal organization that supports viral assembly, as seen in mammalian reovirus (MRV) infections (Liu, X. et al., 2024). Similarly, the structure of stress granules and P-bodies, involved in mRNA triage and decay, are being elucidated in their cellular milieu, revealing their interactions with ribosomes and the endoplasmic reticulum.
Technological advancements are continually pushing the boundaries of what cryo-ET can achieve. Cryo-FIB milling sculpts vitrified cells into thin, electron-transparent lamellae suitable for tomography. Innovations like serialized on-grid lift-in sectioning for tomography (SOLIST) (Nguyen, H.T.D. et al., 2024) and Serial Lift-Out (Schiøtz, O.H. et al., 2023) are enhancing throughput and allowing for the examination of condensates in more complex samples like tissues and even small organisms. Furthermore, genetically encoded multimeric tags (GEMs) that are visible in both fluorescence microscopy and cryo-ET are improving the specific localization and identification of target proteins within condensates (Fung, H.K.H. et al., 2023).
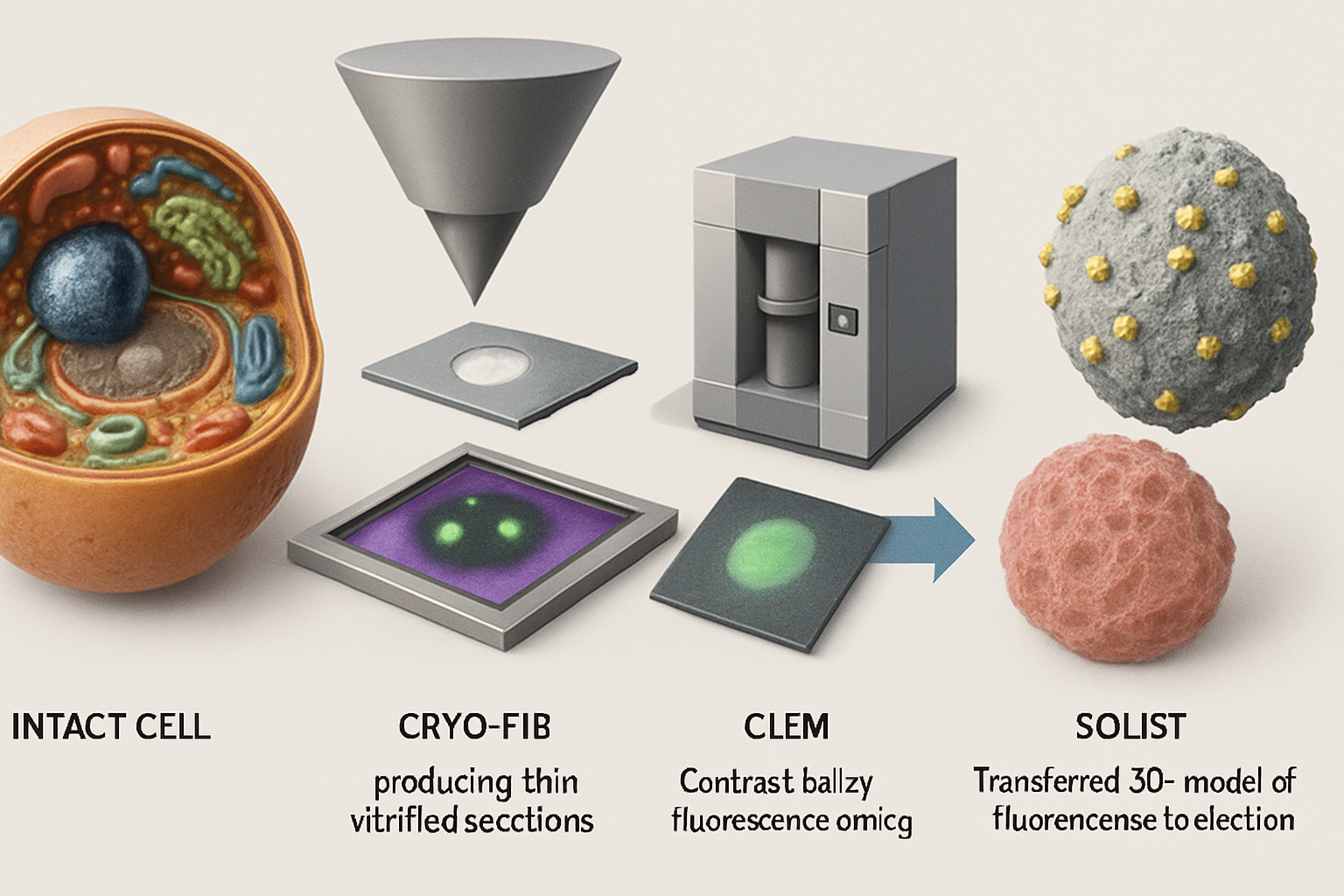
These tools are crucial for visualizing how condensates, such as those involved in chromatin organization (Chen, J.K. et al., 2025; Uckelmann, M. et al., 2024) or polyphosphate storage (Chawla, R. et al., 2024), engage with other cellular structures like the cytoskeleton or organelle membranes.
Molecular Mechanisms of Condensate Assembly and Material States
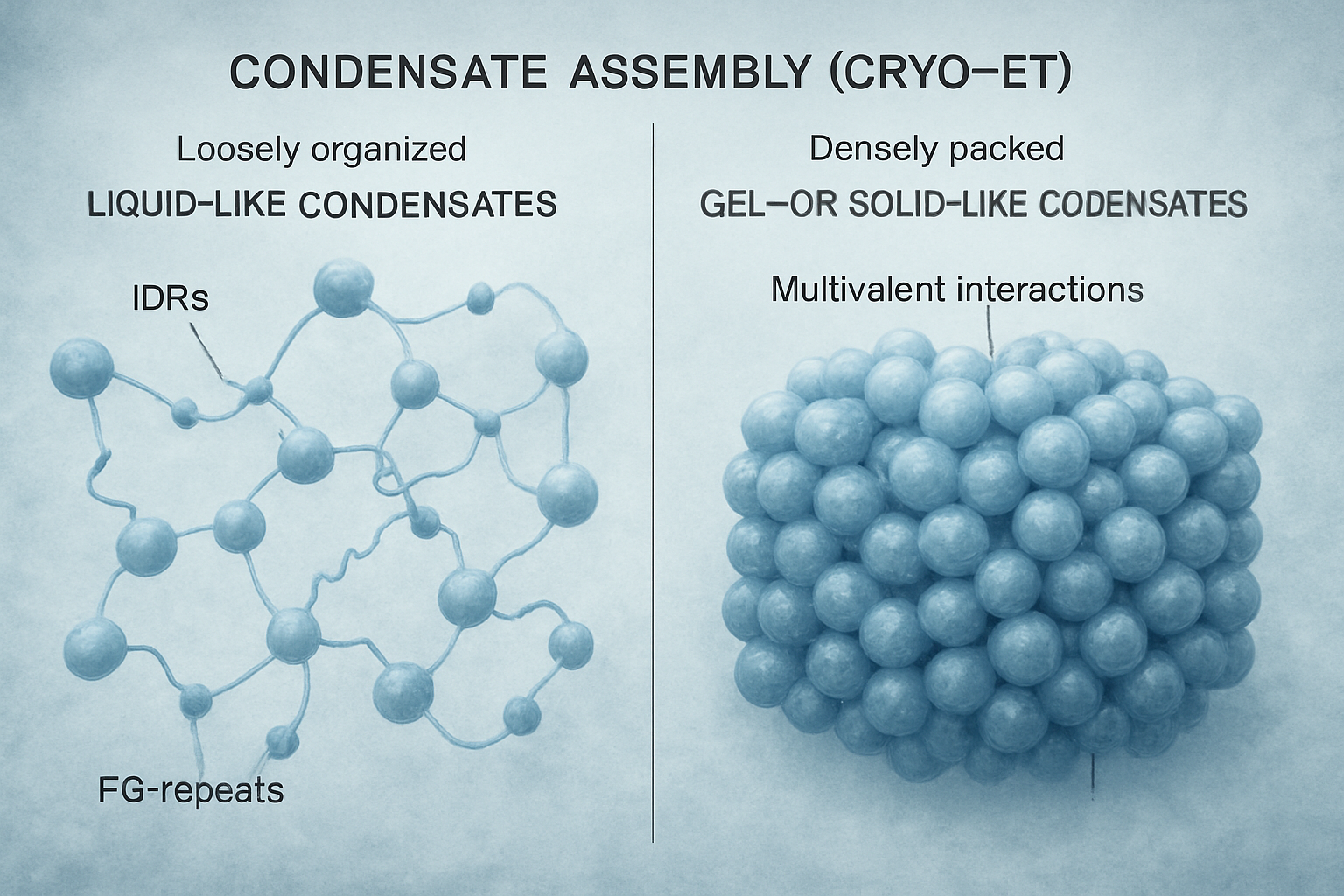
Cryo-ET provides direct visual evidence for the molecular interactions that drive condensate formation and determine their material properties. The multivalency of protein interactions, often mediated by intrinsically disordered regions (IDRs) and specific motifs like phenylalanine-glycine (FG) repeats in nucleoporins (Ibáñez de Opakua, A. et al., 2024; Ng, S.C. et al., 2023), are key drivers of LLPS. Cryo-ET can visualize the resulting supramolecular assemblies, from loosely organized networks to more densely packed structures. For example, studies on Nup98 FG domains show how spacer length and motif identity dictate phase density and barrier properties, crucial for nuclear pore function.
The material state of condensates—ranging from highly dynamic liquids to more stable gels or even solid-like assemblies—is increasingly recognized as a critical functional parameter. Lasker, K. et al. (2022) demonstrated that the material properties of bacterial PopZ condensates, tunable by balancing attractive and repulsive forces, are critical for proper cell division. Cryo-ET, by capturing snapshots of these assemblies, can infer these properties from the packing density and organization of their constituents. Oh, H.J. et al. (2025) even used cryo-ET to visualize the 3D structure of size-controlled protein condensates stabilized by engineered interfacial protein cages, demonstrating how condensate properties can be externally modulated. These studies highlight how the specific molecular makeup and interaction network within a condensate, accessible by cryo-ET, dictate its physical nature and ultimately its biological activity.
Functional Roles and Dysregulation of Condensates Imaged by Cryo-ET
By linking observed structures to cellular activities, cryo-ET powerfully illuminates the functional roles of condensates. For instance, cryo-ET has shown how FMRP granules, implicated in Fragile X syndrome, are recruited to mitochondrial fission sites and locally translate proteins like MFF to regulate mitochondrial dynamics (Fenton, A.R. et al., 2024). In autophagy, phase separation of initiation hubs on cargo surfaces, visualized in yeast and human cells, acts as a trigger switch for selective autophagy (Licheva, M. et al., 2024). The pyrenoid, a CO2-fixing organelle in algae, utilizes matrix-traversing membranes whose biogenesis, involving proteins SAGA1 and MITH1, has been structurally characterized, revealing their role in creating adhesive interactions between membrane and matrix (Hennacy, J.H. et al., 2024).
The technique is also indispensable for understanding how viruses exploit or create condensates. HIV-1, for example, forms nuclear membraneless organelles (HIV-1 MLOs) that persist for weeks, shield the viral genome, and license reverse transcription, as visualized in vivo (Ay, S. et al., 2024). The HIV capsid itself has been shown to mimic karyopherin engagement of FG-nucleoporins to penetrate the nuclear pore condensate (Dickson, C.F. et al., 2023). Similarly, the proteomic analysis of SARS-CoV-2 particles has implicated host stress granule proteins like G3BP1/2 in viral assembly, with their roles likely linked to condensate formation (Murigneux, E. et al., 2024). Aberrant condensate behavior is also central to neurodegenerative diseases. Studies on C9orf72 dipeptide repeat proteins show their aggregation and interaction with molecular chaperones (Liu, F. et al., 2022), and cryo-ET can potentially visualize these pathogenic assemblies in situ.
Generative Insights and Novel Hypotheses from Cryo-ET Studies
Beyond confirming existing models, cryo-ET is a hypothesis-generating engine. The direct visualization of condensate ultrastructure and interactions sparks new ideas about their regulation and function.
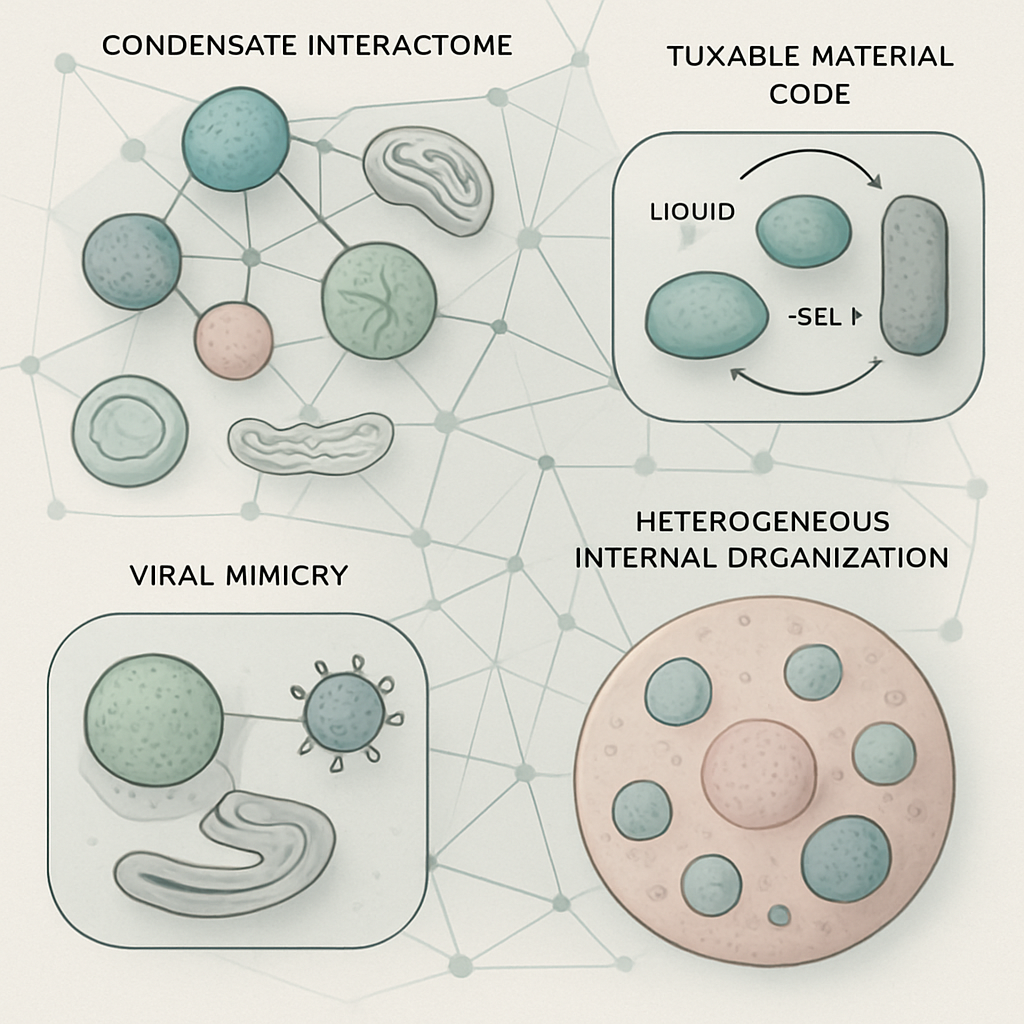
One emerging concept is the "condensate interactome". Cryo-ET reveals that condensates are not isolated entities but are often in close apposition to other organelles (e.g., ER, mitochondria) and even other condensates. We propose that this network of physical and functional interactions forms a higher-order regulatory layer, orchestrating complex cellular responses. For instance, the interface between a stress granule and the ER could facilitate localized translation or stress signaling in ways not possible if they were spatially segregated.
Secondly, the material properties of condensates may function as a dynamic, programmable code. The transition between liquid-like, gel-like, and solid-like states, visualized by differences in molecular packing by cryo-ET (Lasker, K. et al., 2022; Uckelmann, M. et al., 2024), could be actively tuned by the cell to gate molecular access, control reaction kinetics, or alter mechanical properties in response to stimuli. This "material code" might be as important as sequence-specific binding for regulating condensate function.
Thirdly, the phenomenon of viral mimicry of host condensate components, as seen with the HIV capsid and FG-nucleoporins (Dickson, C.F. et al., 2023), might be a widespread viral strategy. We hypothesize that other viruses may have evolved mechanisms to structurally or dynamically mimic components of host condensates, allowing them to co-opt these structures, disrupt their antiviral functions, or use them as havens for replication, camouflaged from immune detection. Cryo-ET is perfectly poised to identify such molecular mimicry at the virus-host condensate interface.
Finally, the internal architecture of condensates, often revealed by cryo-ET to be heterogeneous rather than uniform (Liu, X. et al., 2024), suggests sophisticated internal organization for information processing. Sub-compartments, molecular gradients, or defined interfaces with structures like microtubules could serve as platforms for sequential enzymatic reactions, assembly lines for macromolecular complexes, or channels for vectorial transport of molecules through the condensate, far exceeding simple concentration effects.
Conclusion
Cryo-electron tomography is fundamentally reshaping our understanding of intracellular condensates, providing unparalleled insights into their native architecture, molecular assembly, material properties, and functional integration within the cellular ecosystem. The ability to visualize these dynamic, often transient structures in their unperturbed state is bridging cellular and molecular biology, allowing us to witness biological processes at an unprecedented level of detail.
The ongoing development of cryo-ET methodologies, including improved sample preparation, faster data acquisition, and sophisticated image processing, promises even greater resolving power and the ability to capture dynamic events. Future challenges include imaging rarer or smaller condensates, achieving higher in-cell resolutions to discern individual protein conformations and interactions, and integrating cryo-ET data with complementary 'omics' and live-cell imaging approaches for a truly holistic view. Open questions abound: What is the full complement of condensates within a given cell type? What are the precise rules governing their spatiotemporal assembly and disassembly? How do alterations in condensate structure and dynamics contribute to the full spectrum of human diseases? Cryo-ET will undoubtedly be at the forefront of addressing these questions, continuing to drive conceptual breakthroughs and revealing the intricate, condensed world within our cells.
References
- Ahmed, Y.M. et al. (2024). Phospho-signaling couples polar asymmetry and proteolysis within a membraneless microdomain in Caulobacter crescentus. Nature Communications. https://doi.org/10.1038/s41467-024-53395-y
- Ay, S. et al. (2024). In vivo HIV-1 nuclear condensates safeguard against cGAS and license reverse transcription. The EMBO Journal. https://doi.org/10.1038/s44318-024-00316-w
- Chawla, R. et al. (2024). Reentrant DNA shells tune polyphosphate condensate size. Nature Communications. https://doi.org/10.1038/s41467-024-53469-x
- Chen, J.K. et al. (2025). Nanoscale analysis of human G1 and metaphase chromatin in situ. The EMBO Journal. https://doi.org/10.1038/s44318-025-00407-2
- Conley, M.J. et al. (2021). Helical ordering of envelope‐associated proteins and glycoproteins in respiratory syncytial virus. The EMBO Journal. https://doi.org/10.15252/embj.2021109728
- Dickson, C.F. et al. (2023). The HIV capsid mimics karyopherin engagement of FG-nucleoporins. Nature. https://doi.org/10.1038/s41586-023-06969-7
- Fenton, A.R. et al. (2024). FMRP regulates MFF translation to locally direct mitochondrial fission in neurons. Nature Cell Biology. https://doi.org/10.1038/s41556-024-01544-2
- Fung, H.K.H. et al. (2023). Genetically encoded multimeric tags for subcellular protein localization in cryo-EM. Nature Methods. https://doi.org/10.1038/s41592-023-02053-0
- Hennacy, J.H. et al. (2024). SAGA1 and MITH1 produce matrix-traversing membranes in the CO2-fixing pyrenoid. Nature Plants. https://doi.org/10.1038/s41477-024-01847-0
- Ibáñez de Opakua, A. et al. (2024). Impact of distinct FG nucleoporin repeats on Nup98 self-association. Nature Communications. https://doi.org/10.1038/s41467-024-48194-4
- Kügelgen, A. et al. (2024). Membraneless channels sieve cations in ammonia-oxidizing marine archaea. Nature. https://doi.org/10.1038/s41586-024-07462-5
- Lasker, K. et al. (2022). The material properties of a bacterial-derived biomolecular condensate tune biological function in natural and synthetic systems. Nature Communications. https://doi.org/10.1038/s41467-022-33221-z
- Licheva, M. et al. (2024). Phase separation of initiation hubs on cargo is a trigger switch for selective autophagy. Nature Cell Biology. https://doi.org/10.1038/s41556-024-01572-y
- Liu, F. et al. (2022). Proximity proteomics of C9orf72 dipeptide repeat proteins identifies molecular chaperones as modifiers of poly-GA aggregation. Acta Neuropathologica Communications. http://dx.doi.org/10.1186/s40478-022-01322-x
- Liu, X. et al. (2024). Molecular sociology of virus-induced cellular condensates supporting reovirus assembly and replication. Nature Communications. https://doi.org/10.1038/s41467-024-54968-7
- Maan, R. et al. (2022). Multivalent interactions facilitate motor-dependent protein accumulation at growing microtubule plus-ends. Nature Cell Biology. https://doi.org/10.1038/s41556-022-01037-0
- Martin-Solana, E. et al. (2024). Disruption of the mitochondrial network in a mouse model of Huntington's disease visualized by in-tissue multiscale 3D electron microscopy. Acta Neuropathologica Communications. https://doi.org/10.1186/s40478-024-01802-2
- Murigneux, E. et al. (2024). Proteomic analysis of SARS-CoV-2 particles unveils a key role of G3BP proteins in viral assembly. Nature Communications. https://doi.org/10.1038/s41467-024-44958-0
- Musacchio, A. (2021). On the role of phase separation in the biogenesis of membraneless compartments. The EMBO Journal. https://doi.org/10.15252/embj.2021109952
- Ng, S.C. et al. (2023). Barrier properties of Nup98 FG phases ruled by FG motif identity and inter-FG spacer length. Nature Communications. https://doi.org/10.1038/s41467-023-36331-4
- Nguyen, H.T.D. et al. (2024). Serialized on-grid lift-in sectioning for tomography (SOLIST) enables a biopsy at the nanoscale. Nature Methods. https://doi.org/10.1038/s41592-024-02384-6
- Nieweglowska, E.S. et al. (2023). The φPA3 phage nucleus is enclosed by a self-assembling 2D crystalline lattice. Nature Communications. https://doi.org/10.1038/s41467-023-36526-9
- Oh, H.J. et al. (2025). Size-controlled assembly of phase separated protein condensates with interfacial protein cages. Nature Communications. https://doi.org/10.1038/s41467-025-56391-y
- Schiøtz, O.H. et al. (2023). Serial Lift-Out: sampling the molecular anatomy of whole organisms. Nature Methods. https://doi.org/10.1038/s41592-023-02113-5
- Tillu, V.A. et al. (2021). Cavin1 intrinsically disordered domains are essential for fuzzy electrostatic interactions and caveola formation. Nature Communications. https://doi.org/10.1038/s41467-021-21035-4
- Uckelmann, M. et al. (2024). Dynamic PRC1–CBX8 stabilizes a porous structure of chromatin condensates. Nature Structural & Molecular Biology. https://doi.org/10.1038/s41594-024-01457-6
- Wang, B. et al. (2021). Liquid–liquid phase separation in human health and diseases. Signal Transduction and Targeted Therapy. https://doi.org/10.1038/s41392-021-00678-1
- Wang, H. et al. (2023). Hierarchical organization and assembly of the archaeal cell sheath from an amyloid-like protein. Nature Communications. https://doi.org/10.1038/s41467-023-42368-2
- Winogradoff, D. et al. (2022). Percolation transition prescribes protein size-specific barrier to passive transport through the nuclear pore complex. Nature Communications. https://doi.org/10.1038/s41467-022-32857-1
- Wong, L.E. et al. (2020). Tripartite phase separation of two signal effectors with vesicles priming B cell responsiveness. Nature Communications. https://doi.org/10.1038/s41467-020-14544-1
- Xia, S. et al. (2021). Higher-order assemblies in immune signaling: supramolecular complexes and phase separation. Protein & Cell. https://doi.org/10.1007/s13238-021-00839-6
- Yang, Y. et al. (2023). Nuclear transport proteins: structure, function and disease relevance. Signal Transduction and Targeted Therapy. https://doi.org/10.1038/s41392-023-01649-4
- Zang, K. et al. (2021). Scaffolding protein CcmM directs multiprotein phase separation in β-carboxysome biogenesis. Nature Structural & Molecular Biology. https://doi.org/10.1038/s41594-021-00676-5
- Zhang, M. et al. (2024). Angle between DNA linker and nucleosome core particle regulates array compaction revealed by individual-particle cryo-electron tomography. Nature Communications. https://doi.org/10.1038/s41467-024-48305-1