Bio-integrated Photonics for Neural Interfacing: Illuminating the Path to Understanding and Treating the Brain
The human brain, with its staggering complexity of interconnected neurons, remains one of science's most profound frontiers. Unraveling its intricate communication pathways and understanding the basis of neurological function and dysfunction necessitate tools that can interface with neural circuits with high precision and minimal invasiveness. Traditional electrophysiological methods, while foundational, face limitations in terms of cellular specificity, invasiveness, and the extent of tissue response. Bio-integrated photonics, leveraging the power of light, has emerged as a transformative approach, offering unprecedented spatiotemporal resolution, the ability to target genetically specified cell types, and a diverse range of interaction modalities with neural tissue.
This field is witnessing rapid advancements driven by innovations in materials science, nanofabrication, and optical engineering, propelling the development of sophisticated neural probes that are flexible, biocompatible, and increasingly multifunctional. The ultimate goal is to create seamless, chronic interfaces that can both monitor and modulate neural activity with high fidelity, opening new avenues for fundamental neuroscience research and the treatment of debilitating neurological and psychiatric disorders.
This article reviews the cutting-edge developments in bio-integrated photonics for neural interfacing. We will explore the foundational materials and fabrication strategies enabling the intimate integration of light-based technologies with delicate neural tissues, delve into the diverse photonic modalities used for neural interrogation and modulation, and discuss the push towards chronic, intelligent, and wireless photonic neural interfaces that promise to revolutionize our ability to interact with the nervous system.
Foundations of Bio-integrated Photonics: Materials and Fabrication
The success of any implantable neural interface hinges on its ability to integrate harmoniously with the biological environment while maintaining robust functionality. For photonic devices, this requires materials that are not only optically transparent and efficient at the wavelengths of interest but also mechanically compliant with soft neural tissue, biocompatible to minimize inflammatory responses, and stable for long-term operation, or controllably biodegradable if transient use is intended.
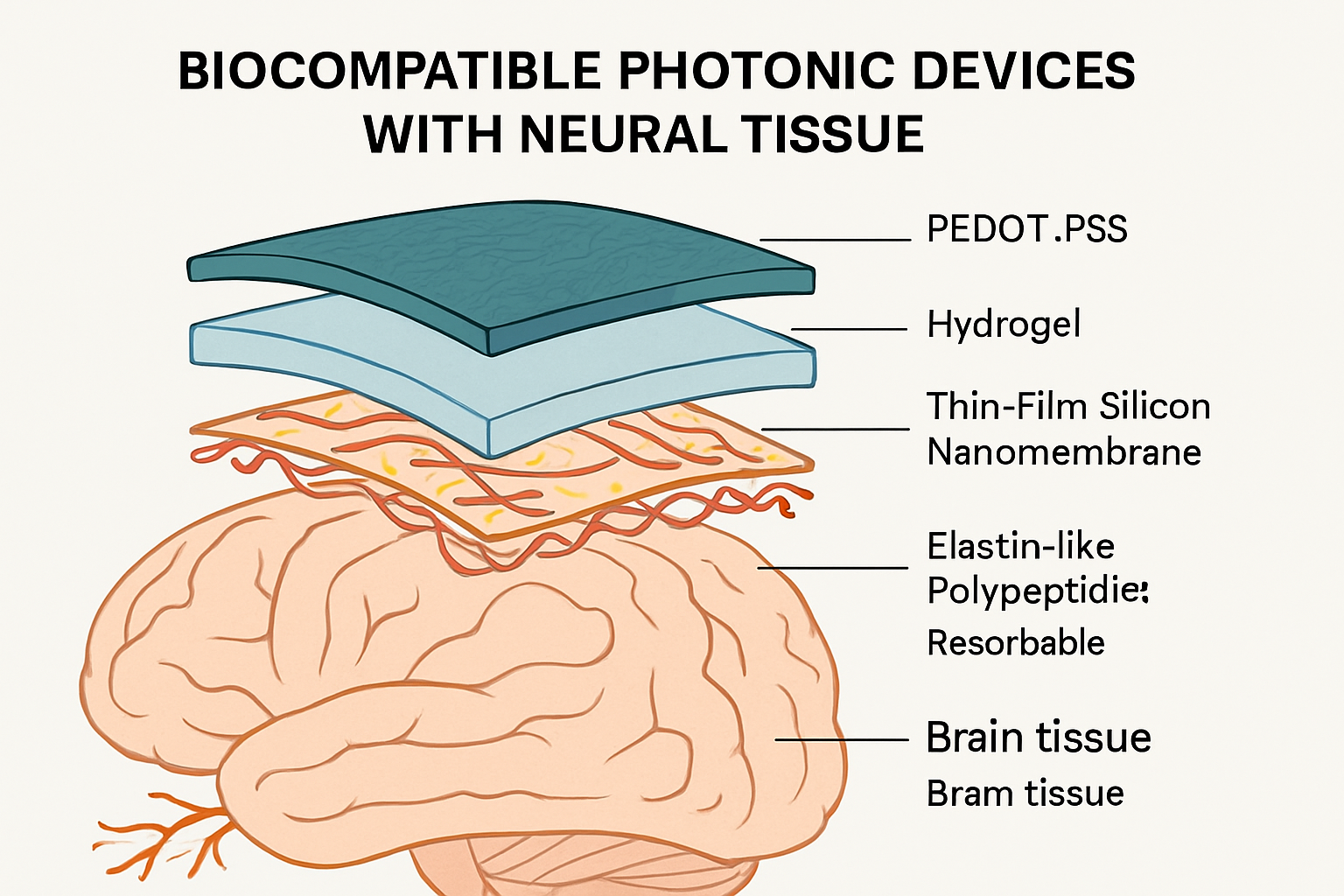
Biocompatible and Flexible Materials: To mitigate mechanical mismatch and associated chronic tissue damage, researchers are increasingly turning to soft, flexible materials. Polymers such as PEDOT:PSS are favored for their conductivity and processability in creating flexible bioelectrodes, which can be combined with optical components. Stretchable optoelectronics allow devices to conform and move with tissue, crucial for applications like retina-inspired computing. Hydrogels and elastomers are also explored for their tissue-like softness and potential for drug delivery.
Bioresorbable Photonics: A paradigm-shifting approach involves creating devices that perform their function for a desired period and then safely dissolve in the body, eliminating the need for risky explantation surgeries and reducing long-term chronic foreign body reactions. Such systems use biodegradable polymers, thin-film silicon nanomembranes, and specially engineered biobased photoresists.
Advanced Fabrication and Integration: Fabrication processes often involve sophisticated micro and nanofabrication techniques to create waveguides, optical gratings, and integrate light sources like micro-LEDs. Advances also include 3D printing of micro-nano devices and the use of novel adhesives and mechanical structures for stable bio-integration.
Photonic Modalities for Neural Interrogation and Modulation
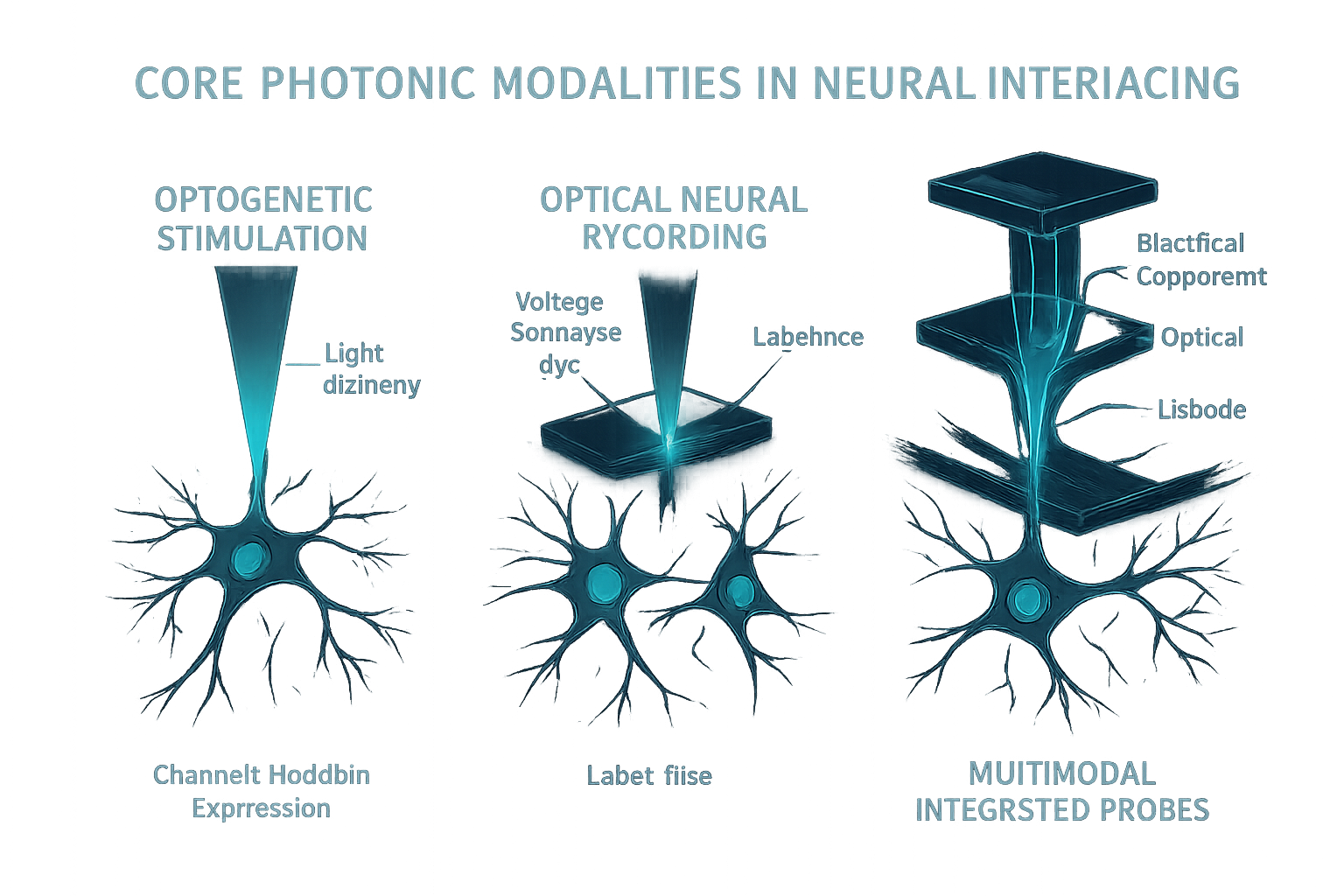
Light offers a versatile toolkit for interacting with neurons, from precisely controlling their activity to potentially reading out their signaling patterns. Optogenetics, which uses genetically introduced light-sensitive proteins to control neuronal firing, has been a primary driver for bio-integrated photonics. Delivering light deep within the brain with cellular and network-level precision is critical, enabled by implantable nanophotonic neural probes and flexible silicon-based phased arrays for dynamic light delivery.
Optical Neural Recording: While optical stimulation is well-established, robust, direct optical recording of neural activity with high spatiotemporal resolution—especially for chronic applications—remains a major challenge. Current multimodal probes combine optical stimulation with traditional electrophysiological recording, while novel sensor materials and label-free optical methods show promise for the future.
Multimodal Interfaces: The integration of multiple modalities is already mature, with devices combining optical stimulation, electrical recording, and microfluidics for localized drug delivery and biochemical analysis. These advances are enabling more comprehensive and minimally invasive interrogation and modulation of neural circuits.
Towards Chronic and Intelligent Photonic Neural Interfaces
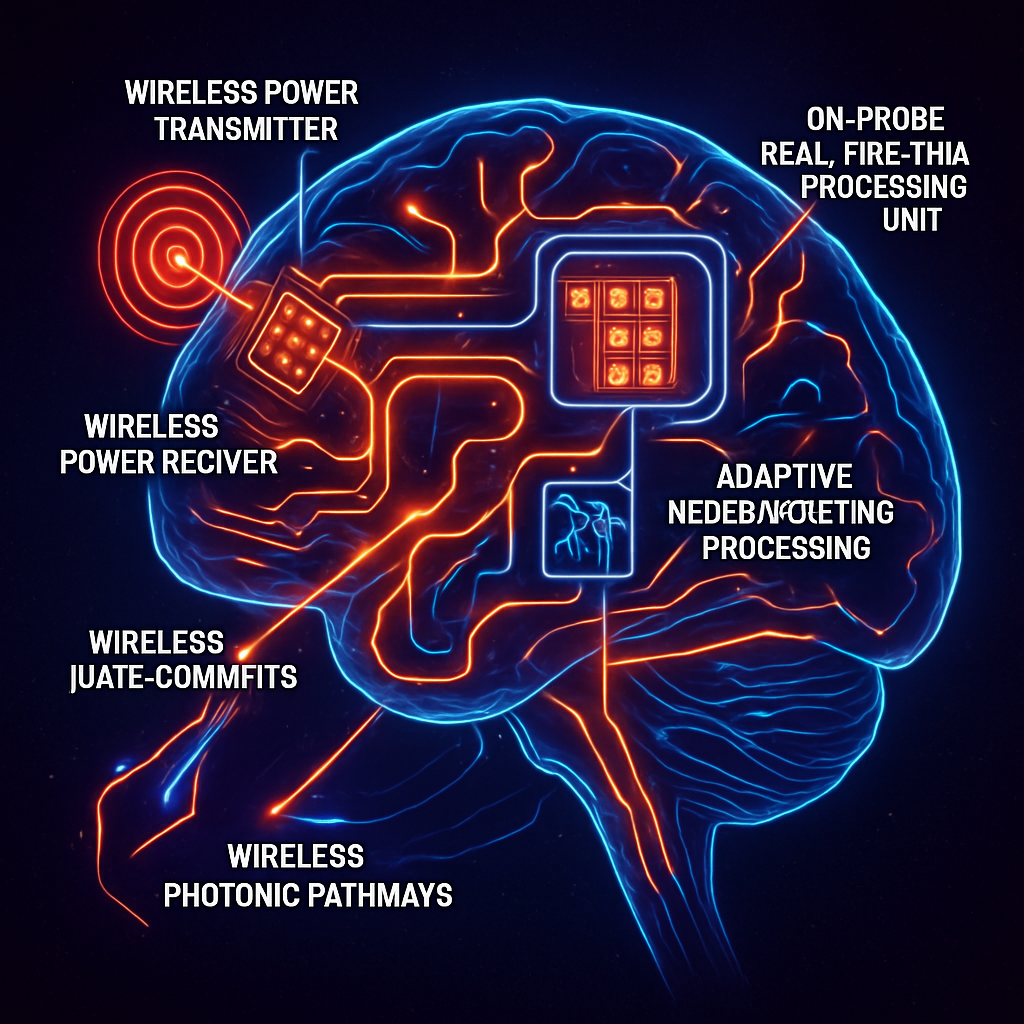
The ultimate aim is to develop photonic neural interfaces that can operate reliably for extended periods, ideally wirelessly, and adapt to the changing neural environment. Tethers restrict natural behavior and limit neuroscience experiments, so wireless power and data transmission are crucial for chronic studies. Implantable systems now leverage wireless technologies for both power and control.
Addressing Long-Term Stability and Biocompatibility: The foreign body response remains a hurdle, leading to scarring and device failure. Strategies to enhance long-term stability include biocompatible, compliant materials, optimal device geometries, surface functionalization, and the use of bioresorbable materials to address chronic responses altogether.
Integration with Neuromorphic and Closed-Loop Systems: Advanced neural interfaces may incorporate on-board intelligence, sensing neural states and adapting stimulation in real-time. Stretchable optoelectronic synapses and flexible neuromorphic sensors portend the development of adaptive bio-integrated systems with real-time computational capabilities.
Conclusion
Bio-integrated photonics is rapidly transforming our ability to interact with the nervous system. Remarkable progress has been made in developing flexible, bioresorbable, wireless, and multimodal photonic neural interfaces. These tools are not only advancing fundamental neuroscience by enabling precise optogenetic control and paving the way for novel recording modalities but also hold significant promise for new therapeutic interventions for neurological and psychiatric disorders.
However, significant challenges remain. Achieving true, high-fidelity optical sensing of diverse neural states (voltage, specific neurotransmitters) in chronically implanted devices is a major frontier. Ensuring the lifelong stability and biocompatibility of non-resorbable implants, or precisely controlling the degradation and biological interaction of resorbable ones, requires continued innovation in materials science and bioengineering. Furthermore, the sheer volume of data generated by high-resolution interfaces will necessitate advanced data processing and interpretation strategies.
Looking ahead, we propose a provocative hypothesis: The full potential of bio-integrated photonics for neural interfacing will be unlocked through the convergence of adaptive nanophotonics, intelligent biomaterials that actively respond to their environment, and on-probe neuromorphic processing capabilities. Imagine devices that can dynamically reconfigure their optical interrogation and stimulation patterns in response to real-time neural feedback or subtle changes in the tissue microenvironment, learning and adapting to optimize therapeutic outcomes or research insights. The journey to seamlessly merging light-based technology with the intricacies of neural circuits is complex but laden with the promise of profound discoveries and life-changing applications.
References
- Cho, M. et al. (2024). Fully bioresorbable hybrid opto-electronic neural implant system for simultaneous electrophysiological recording and optogenetic stimulation. Nature Communications. https://doi.org/10.1038/s41467-024-45803-0
- Chen, F-D. et al. (2025). Implantable nanophotonic neural probes for integrated patterned photostimulation and electrophysiological recording. npj Biosensing. https://doi.org/10.1038/s44328-025-00024-3
- Huang, Y. et al. (2024). Flexible electronic-photonic 3D integration from ultrathin polymer chiplets. npj Flexible Electronics. https://doi.org/10.1038/s41528-024-00344-w
- Sahasrabudhe, A. et al. (2023). Multifunctional microelectronic fibers enable wireless modulation of gut and brain neural circuits. Nature Biotechnology. https://doi.org/10.1038/s41587-023-01833-5
- Li, J. et al. (2025). PEDOT:PSS-based bioelectronics for brain monitoring and modulation. Microsystems & Nanoengineering. https://doi.org/10.1038/s41378-025-00948-w
- Lan, L. et al. (2025). Stretchable optoelectronic synapses with ultraviolet to near-infrared perception for retina-inspired computing and vision-adaptive sensing. npj Flexible Electronics. https://doi.org/10.1038/s41528-025-00390-y
- Kim, K. et al. (2023). Fully implantable and battery-free wireless optoelectronic system for modulable cancer therapy and real-time monitoring. npj Flexible Electronics. https://doi.org/10.1038/s41528-023-00276-x
- Silva, P. & Jacinto, L. (2024). Wireless Devices for Optical Brain Stimulation: A Review of Current Developments for Optogenetic Applications in Freely Moving Mice. Cellular and Molecular Bioengineering. https://doi.org/10.1007/s12195-024-00832-z
- Lv, S. et al. (2025). Long-term stability strategies of deep brain flexible neural interface. npj Flexible Electronics. https://doi.org/10.1038/s41528-025-00410-x
- Dalrymple, A.N. et al. (2025). Overcoming failure: improving acceptance and success of implanted neural interfaces. Bioelectronic Medicine. https://doi.org/10.1186/s42234-025-00168-7
- Chen, F-D. et al. (2024). Implantable silicon neural probes with nanophotonic phased arrays for single-lobe beam steering. Communications Engineering. https://doi.org/10.1038/s44172-024-00328-8
- Balogh-Lantos, Zs. et al. (2024). High density laminar recordings reveal cell type and layer specific responses to infrared neural stimulation in the rat neocortex. Scientific Reports. https://doi.org/10.1038/s41598-024-82980-w
- Seo, S.J. & Jang, H.W. (2025). Flexible Micro-LEDs: Advanced Fabrication Techniques and Applications. Electronic Materials Letters. https://doi.org/10.1007/s13391-025-00559-7
- Zhou, M. et al. (2025). Improving multifunctional monolithic nano-device by single (Al,Ga)N nanowire/graphene van der Waals heterostructure for neuromorphic computing, photodetection and imaging. Communications Materials. https://doi.org/10.1038/s43246-025-00803-5
- Joseph, E. et al. (2024). Photovoltaic bioelectronics merging biology with new generation semiconductors and light in biophotovoltaics photobiomodulation and biosensing. npj Biosensing. https://doi.org/10.1038/s44328-024-00015-w
- Martino, N. et al. (2025). Large-scale combinatorial optical barcoding of cells with laser particles. Light: Science & Applications. https://doi.org/10.1038/s41377-025-01809-x
- Pritzl, S.D. et al. (2025). Photoswitchable phospholipids for the optical control of membrane processes, protein function, and drug delivery. Communications Materials. https://doi.org/10.1038/s43246-025-00773-8
- Hossain, M.J. et al. (2024). Additive Manufacturing of Sensors: A Comprehensive Review. International Journal of Precision Engineering and Manufacturing-Green Technology. https://doi.org/10.1007/s40684-024-00629-5
- Zhang, N. et al. (2024). 3D printing of micro-nano devices and their applications. Microsystems & Nanoengineering. https://doi.org/10.1038/s41378-024-00812-3
- Lerman, I. et al. (2024). Next generation bioelectronic medicine: making the case for non-invasive closed-loop autonomic neuromodulation. Bioelectronic Medicine. https://doi.org/10.1186/s42234-024-00163-4
- Gutruf, P. (2023). Towards a digitally connected body for holistic and continuous health insight. Communications Materials. https://doi.org/10.1038/s43246-023-00443-7
- Wei, R. et al. (2024). Revolutionizing wearable technology: advanced fabrication techniques for body-conformable electronics. npj Flexible Electronics. https://doi.org/10.1038/s41528-024-00370-8
- Geng, Z. et al. (2024). Material engineering and application of hybrid biomimetic-de novo designed elastin-like polypeptides. Communications Materials. https://doi.org/10.1038/s43246-024-00597-y
- Sun, Y. et al. (2024). Wearable Biodevices Based on Two-Dimensional Materials: From Flexible Sensors to Smart Integrated Systems. Nano-Micro Letters. https://doi.org/10.1007/s40820-024-01597-w
- Thomas, K. et al. (2024). GABAergic neurons in basal forebrain exert frequency-specific modulation on auditory cortex and enhance attentional selection of auditory stimuli. Communications Biology. https://doi.org/10.1038/s42003-024-07318-8
- Kim, J. et al. (2025). Smart Mechanical Structures and Design for Advanced Adhesives: A Review. International Journal of Precision Engineering and Manufacturing. https://doi.org/10.1007/s12541-025-01211-y
- Lim, K. et al. (2024). Material and structural considerations for high-performance electrodes for wearable skin devices. Communications Materials. https://doi.org/10.1038/s43246-024-00490-8
- Wang, H. et al. (2024). On non-von Neumann flexible neuromorphic vision sensors. npj Flexible Electronics. https://doi.org/10.1038/s41528-024-00313-3
- Sun, T. et al. (2023). Artificial Intelligence Meets Flexible Sensors: Emerging Smart Flexible Sensing Systems Driven by Machine Learning and Artificial Synapses. Nano-Micro Letters. https://doi.org/10.1007/s40820-023-01235-x
- Stevens, A.R. et al. (2025). Evaluation of transcriptomic changes after photobiomodulation in spinal cord injury. Scientific Reports. https://doi.org/10.1038/s41598-025-87300-4