Astrovirology: Investigating Viral Biosignatures on Other Worlds

Astrobiology, the study of the origin, evolution, distribution, and future of life in the universe, has traditionally focused on cellular life forms. However, on Earth, viruses are the most abundant biological entities, profoundly influencing microbial ecosystems, global biogeochemical cycles, and the evolution of cellular life itself. The emerging field of astrovirology proposes that the search for extraterrestrial life should encompass viruses and virus-like particles (VLPs). Ignoring these entities may mean overlooking a fundamental aspect of any biosphere, terrestrial or otherwise. This article explores the rationale for astrovirology, examines potential viral biosignatures, discusses detection challenges and strategies, and speculates on the role viruses might play on other worlds.
The consideration of viruses in astrobiology is supported by their ubiquity and resilience in Earth's most extreme environments, which serve as analogues for potentially habitable locations elsewhere in the solar system. From the icy brine channels of polar sea ice to hydrothermal vents, hypersaline lakes, and deep subsurface rocks, viruses thrive where cellular life exists. Furthermore, the unique nature of viruses—existing at the boundary of living and non-living, their potential role in the origin of life, and their capacity for rapid evolution and horizontal gene transfer—makes them compelling targets in the quest to understand life beyond Earth.
Viruses in Extreme Environments as Terrestrial Analogs

The ability of terrestrial life to inhabit extreme environments provides a crucial baseline for assessing the habitability of other planets and moons. Viruses are integral components of these extremophile communities. Studies have identified diverse and often novel viral populations in hypersaline environments (Oren, 2024), deep-sea hydrothermal vents, acidic geothermal springs, oligotrophic cave pools (Ulbrich et al., 2024), Antarctic ecosystems (Zucconi et al., 2025), and Arctic sea ice (Lund-Hansen et al., 2024). Sea ice brine channels, for instance, harbor viruses adapted to temperatures down to -25°C and high salinity, offering analogues for icy moons like Europa or Enceladus (Lund-Hansen et al., 2024). Similarly, anoxic, stratified lakes hosting purple and green sulfur bacteria, analogous to early Earth oceans, show distinct viral activity patterns, including reduced lysis in dense microbial plates, suggesting shifts towards lysogeny or other interaction modes in such environments (Varona et al., 2025).
The survival strategies of extremophile viruses, often involving unique capsid structures, genetic adaptations, and interactions like lysogeny (integrating into the host genome), are relevant to astrovirology. Lysogeny might allow viruses to persist through harsh conditions or resource scarcity, potentially leaving detectable genomic signatures within host genomes (Varona et al., 2025; Song et al., 2025). Furthermore, studies on microbes isolated from spacecraft assembly facilities (Leo et al., 2023; Chander et al., 2024) and the International Space Station (Szydlowski et al., 2024) demonstrate the capacity for some microbes (and potentially associated viruses or their components) to withstand desiccated, irradiated conditions relevant to interplanetary transit or planetary surfaces, highlighting considerations for both planetary protection and the potential for panspermia.
Potential Viral Biosignatures
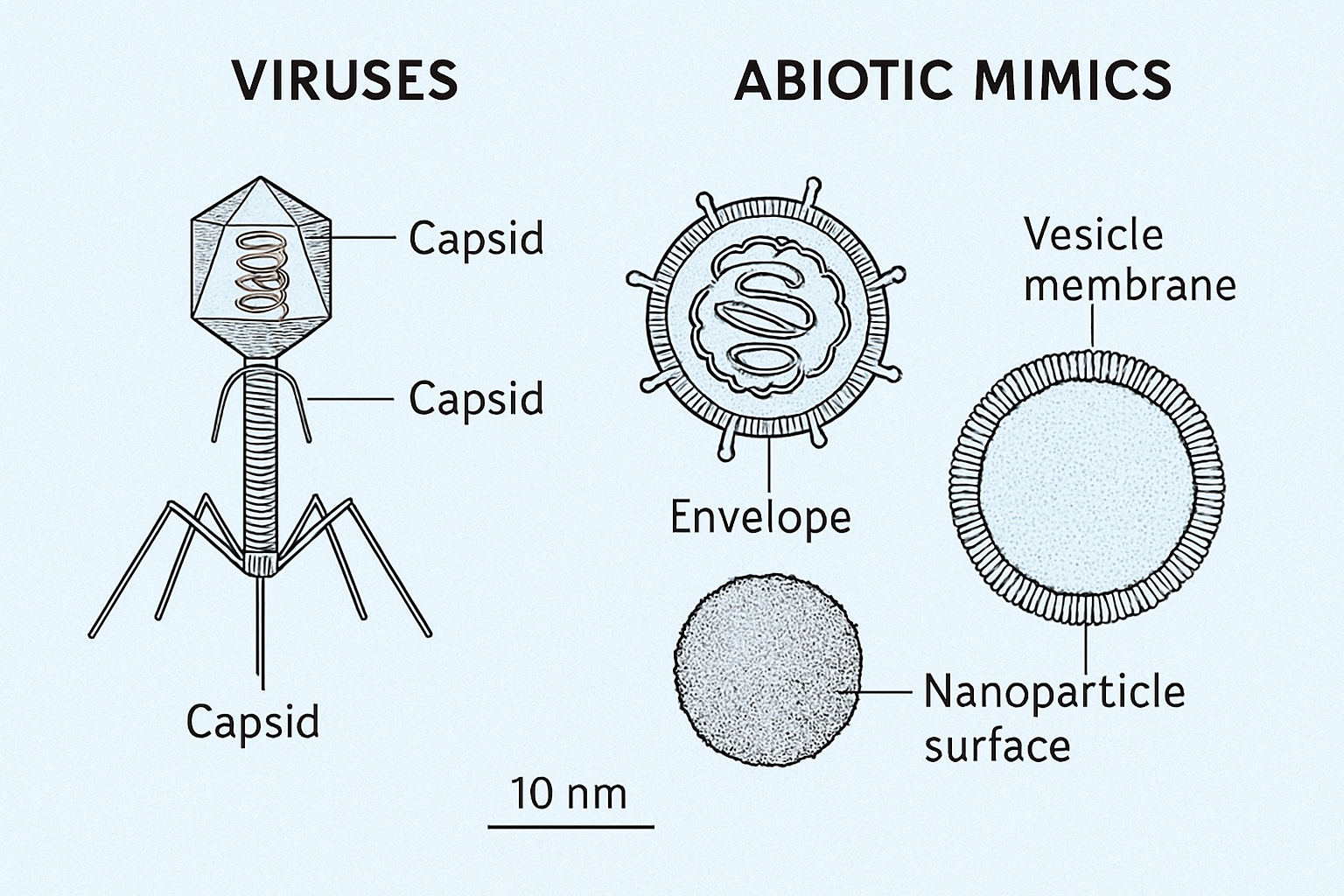
Identifying potential viral biosignatures requires considering features distinct from cellular life or abiotic particles. Potential signatures include:
- Morphological Evidence: Viruses possess characteristic nanoscale structures (e.g., icosahedral capsids, helical nucleocapsids, tailed bacteriophages) with specific symmetries and size ranges (typically 20-400 nm, though giant viruses can reach micron scale). Detection would likely require high-resolution microscopy techniques, such as transmission electron microscopy (TEM) or atomic force microscopy (AFM), adapted for in situ planetary science (Enya et al., 2022). Distinguishing these from similarly sized abiotic mineral formations or cellular debris would require contextual information and ideally correlative chemical analysis.
- Genetic Material: Viral genomes (DNA or RNA, single or double-stranded) contain genes essential for replication and virion assembly, often lacking homologs in cellular life or exhibiting unique genomic architectures. Conserved viral hallmark genes (e.g., capsid proteins, packaging ATPases) or unique genetic sequences identified through metagenomic sequencing could serve as biosignatures. Detecting RNA viruses poses stability challenges, but RNA has been recovered from ancient terrestrial samples. The presence of viral sequences integrated into host genomes (proviruses) could also be a durable biosignature.
- Chemical Composition: Viral capsids are proteinaceous structures, offering a potential chemical biosignature detectable via mass spectrometry or specific binding assays (e.g., antibodies, aptamers). Some viruses possess lipid envelopes derived from host cells but potentially modified or containing unique viral proteins. Specific lipid profiles or proteinaceous patterns distinct from expected cellular or abiotic sources could be indicative.
- Activity or Impact: Viruses actively replicate within host cells, potentially leaving indirect evidence. Detection of specific viral enzymatic activities (e.g., polymerases, lysozymes) or metabolic impacts via Auxiliary Metabolic Genes (AMGs) that redirect host metabolism (Ulbrich et al., 2024; Varona et al., 2025; Song et al., 2025) might be possible, though technically challenging for remote missions. Patterns of host lysis or specific isotopic fractionation linked to viral activity could also be considered.
Establishing the biogenicity of any potential viral signature would require stringent criteria, ideally involving multiple lines of evidence. Differentiating complex VLPs from simple abiotic nanoparticles or membrane vesicles shed by cells remains a significant challenge. Contamination control, as emphasized in planetary protection protocols (Danko et al., 2021), is paramount.
Detection Strategies for Extraterrestrial Viruses
Detecting viral biosignatures on other worlds necessitates sensitive, robust instrumentation capable of operating remotely or analyzing returned samples. Potential approaches include:
- Advanced Microscopy: Miniaturized electron microscopes, AFMs, or advanced light microscopy techniques like fluorescence microscopy (potentially using dyes binding to nucleic acids or proteins) could provide morphological evidence (Enya et al., 2022). Correlative microscopy linking morphology with chemical composition (e.g., Raman spectroscopy, EDX) would strengthen interpretations.
- Nucleic Acid Sequencing: Portable sequencing technologies (e.g., nanopore sequencing) adapted for space conditions could detect viral genomes or transcripts in environmental samples (water, ice, regolith). Challenges include low biomass, nucleic acid degradation (especially RNA), and distinguishing signal from contamination or terrestrial analogues (Maggiori et al., 2021). Functional metagenomics approaches could potentially identify genes conferring survival advantages (Roberts Kingman et al., 2024).
- Mass Spectrometry: Instruments like Gas Chromatography-Mass Spectrometry (GC-MS) or Laser Desorption/Ionization Mass Spectrometry (LDMS) aboard landers or orbiters could search for specific viral proteins, lipids, or breakdown products. High sensitivity and resolution are needed to detect trace amounts and differentiate from abiotic organic molecules.
- Life Detection Assays: Miniaturized assays could target specific viral components (e.g., using antibodies or aptamers) or enzymatic activities. Developing agnostic methods that detect repeating polymers (like proteins or nucleic acids) or characteristic chirality might capture both viral and cellular signatures.
Upcoming missions like Europa Clipper (Vance et al., 2023) and potential future sample return missions from Mars or ocean worlds offer opportunities to search for diverse biosignatures, potentially including viral ones. Integrating astrovirology goals into mission planning and instrument development is crucial.
Viruses, the Origin of Life, and Exobiology
Viruses occupy a unique position in discussions about the origin and definition of life. Their reliance on host cells for replication complicates their classification as 'living,' yet their evolutionary dynamics and complex structures clearly distinguish them from inanimate matter. Some hypotheses posit that virus-like entities, perhaps related to self-replicating RNA molecules or proteinaceous structures, played a role in the transition from non-life to life (abiogenesis). Could VLPs represent a universal intermediate stage or byproduct of emerging life? Exploring the deep phylogeny and origins of terrestrial viruses, including debates on RNA vs. DNA genomes in early life (Cottom-Salas et al., 2024), provides context for what might be possible elsewhere.
If viruses were discovered on another world, it would have profound implications. Their presence alongside cellular life might suggest co-evolution, perhaps indicating a universal role for viruses in driving genetic innovation and ecological regulation. Finding viruses or VLPs in the absence of detectable cellular life would be even more provocative, potentially pointing towards an independent origin, relics of a past cellular biosphere, or a pre-cellular stage of life. The Copernican principle suggests that terrestrial life, including its viral component, might not be special but representative (Hegner, 2024). Thus, searching for entities structurally and functionally similar to terrestrial viruses is a rational starting point. Their simpler structure compared to cells might even make them more likely to arise or persist under certain conditions.
Conclusion
Astrovirology represents a necessary expansion of astrobiology's scope. Viruses are a dominant feature of Earth's biosphere, driving evolution and biogeochemistry, and thriving in environments analogous to those found elsewhere in the solar system. Potential viral biosignatures range from morphology and genetics to chemical composition, though detection and confirmation face significant hurdles, including differentiation from abiotic mimics and terrestrial contamination. Future space missions equipped with advanced analytical instruments, combined with continued exploration of terrestrial extremophile viruses and theoretical modeling, are needed to investigate the possibility of viral existence beyond Earth. Considering viruses alongside cellular life provides a more complete framework for understanding the potential diversity, distribution, and fundamental nature of life in the universe. The discovery of extraterrestrial viruses, in any form, would revolutionize our understanding of biology.
References
- Amin, A. et al. (2024). Resurrected microorganisms: a plethora of resting bacteria underway for human interaction. AMB Express. http://dx.doi.org/10.1186/s13568-024-01750-z
- Bonacolta, A. M. et al. (2024). The eukaryome of modern microbialites reveals distinct colonization across aquatic ecosystems. npj Biofilms and Microbiomes. http://dx.doi.org/10.1038/s41522-024-00547-z
- Borsodi, A. K. (2024). Taxonomic diversity of extremophilic prokaryotes adapted to special environmental parameters in Hungary: a review. Biologia Futura. http://dx.doi.org/10.1007/s42977-024-00224-4
- Breeden, J. L. (2025). The evolution of goals in AI agents. AI and Ethics. http://dx.doi.org/10.1007/s43681-025-00691-y
- Chander, A. M. et al. (2024). Genomic and morphological characterization of Knufia obscura isolated from the Mars 2020 spacecraft assembly facility. Scientific Reports. http://dx.doi.org/10.1038/s41598-024-61115-1
- Cottom-Salas, W. et al. (2024). RNA or DNA? Revisiting the Chemical Nature of the Cenancestral Genome. Journal of Molecular Evolution. http://dx.doi.org/10.1007/s00239-024-10194-9
- Cowan, D. A. et al. (2024). Extremophiles in a changing world. Extremophiles. http://dx.doi.org/10.1007/s00792-024-01341-7
- Danko, D. C. et al. (2021). A comprehensive metagenomics framework to characterize organisms relevant for planetary protection. Microbiome. http://dx.doi.org/10.1186/s40168-021-01020-1
- Enya, K. et al. (2022). Extraterrestrial Life Signature Detection Microscopy: Search and Analysis of Cells and Organics on Mars and Other Solar System Bodies. Space Science Reviews. http://dx.doi.org/10.1007/s11214-022-00920-4
- Glonek, T. (2021). Did Cyclic Metaphosphates Have a Role in the Origin of Life?. Origins of Life and Evolution of Biospheres. http://dx.doi.org/10.1007/s11084-021-09604-5
- Hegner, I. (2024). Terrestrial life in light of the Copernican principle. Discover Life. http://dx.doi.org/10.1007/s11084-024-09659-0
- Hellweg, C. E. et al. (2022). Space Radiobiology. Radiobiology Textbook. http://dx.doi.org/10.1007/978-3-031-18810-7_10
- Inskeep, W. P. et al. (2024). Respiratory processes of early-evolved hyperthermophiles in sulfidic and low-oxygen geothermal microbial communities. Nature Communications. http://dx.doi.org/10.1038/s41467-024-55079-z
- Kailing, F. et al. (2024). Evolution of Cellular Organization Along the First Branches of the Tree of Life. Journal of Molecular Evolution. http://dx.doi.org/10.1007/s00239-024-10188-7
- Koehle, A. P. et al. (2023). Microbial applications for sustainable space exploration beyond low Earth orbit. npj Microgravity. http://dx.doi.org/10.1038/s41526-023-00285-0
- Kuehnast, T. et al. (2021). The crewed journey to Mars and its implications for the human microbiome. Microbiome. http://dx.doi.org/10.1186/s40168-021-01222-7
- Landa, C. R. et al. (2024). Adapting the rhizome concept to an extended definition of viral quasispecies and the implications for molecular evolution. Scientific Reports. http://dx.doi.org/10.1038/s41598-024-68760-6
- Leo, P. et al. (2023). Genomic characterization and radiation tolerance of Naganishia kalamii sp. nov. and Cystobasidium onofrii sp. nov. from Mars 2020 mission assembly facilities. IMA Fungus. http://dx.doi.org/10.1186/s43008-023-00119-4
- Lezcano, M. Á. et al. (2024). Hyperexpansion of genetic diversity and metabolic capacity of extremophilic bacteria and archaea in ancient Andean lake sediments. Microbiome. http://dx.doi.org/10.1186/s40168-024-01878-x
- Lund-Hansen, L. C. et al. (2024). Sea ice as habitat for microalgae, bacteria, virus, fungi, meio- and macrofauna: A review of an extreme environment. Polar Biology. http://dx.doi.org/10.1007/s00300-024-03296-z
- Maggiori, C. et al. (2021). MinION sequencing from sea ice cryoconites leads to de novo genome reconstruction from metagenomes. Scientific Reports. http://dx.doi.org/10.1038/s41598-021-00026-x
- Marchal, S. et al. (2024). Challenges for the human immune system after leaving Earth. npj Microgravity. http://dx.doi.org/10.1038/s41526-024-00446-9
- Moreau, S. J. M. et al. (2025). Multi-omic approach to characterize the venom of the parasitic wasp Cotesia congregata (Hymenoptera: Braconidae). BMC Genomics. http://dx.doi.org/10.1186/s12864-025-11604-y
- Mustafa, O. et al. (2025). A review of the occurrence, fate, and transport of SARS‑CoV‑2 in the aqueous environment, with specific reference to groundwater. Environmental Earth Sciences. http://dx.doi.org/10.1007/s12665-025-12256-7
- Oren, A. (2024). Novel insights into the diversity of halophilic microorganisms and their functioning in hypersaline ecosystems. npj Biodiversity. http://dx.doi.org/10.1038/s44185-024-00050-w
- Roberts Kingman, G. A. et al. (2024). Raiding nature’s genetic toolbox for UV-C resistance by functional metagenomics. Scientific Reports. http://dx.doi.org/10.1038/s41598-024-83952-w
- Schultz, J. et al. (2023). Life on the Edge: Bioprospecting Extremophiles for Astrobiology. Journal of the Indian Institute of Science. http://dx.doi.org/10.1007/s41745-023-00382-9
- Shen, J. et al. (2023). Renaissance for magnetotactic bacteria in astrobiology. The ISME Journal. http://dx.doi.org/10.1038/s41396-023-01495-w
- Słowakiewicz, M. et al. (2024). Biofilms in modern CaCO3-supersaturated freshwater environments reveal viral proxies. Scientific Reports. http://dx.doi.org/10.1038/s41598-024-75998-7
- Song, X. et al. (2025). Rhizosphere-triggered viral lysogeny mediates microbial metabolic reprogramming to enhance arsenic oxidation. Nature Communications. http://dx.doi.org/10.1038/s41467-025-58695-5
- Szydlowski, L. M. et al. (2024). Adaptation to space conditions of novel bacterial species isolated from the International Space Station revealed by functional gene annotations and comparative genome analysis. Microbiome. http://dx.doi.org/10.1186/s40168-024-01916-8
- Ulbrich, J. et al. (2024). Cave Pools in Carlsbad Caverns National Park Contain Diverse Bacteriophage Communities and Novel Viral Sequences. Microbial Ecology. http://dx.doi.org/10.1007/s00248-024-02479-9
- Vance, S. D. et al. (2023). Investigating Europa’s Habitability with the Europa Clipper. Space Science Reviews. http://dx.doi.org/10.1007/s11214-023-01025-2
- Varona, N. S. et al. (2025). Viral activity in lake analogs of anoxic early Earth oceans. Microbiome. http://dx.doi.org/10.1186/s40168-025-02085-y
- Wagner, N. Y. et al. (2022). Survival strategies of an anoxic microbial ecosystem in Lake Untersee, a potential analog for Enceladus. Scientific Reports. http://dx.doi.org/10.1038/s41598-022-10876-8
- Zucconi, L. et al. (2025). Advocating microbial diversity conservation in Antarctica. npj Biodiversity. http://dx.doi.org/10.1038/s44185-025-00076-8