All-Optical Microfluidics: Leveraging Photothermal Effects to Create Reprogrammable, Non-Invasive Virtual Valves and Pumps for High-Throughput Single-Cell Analysis.
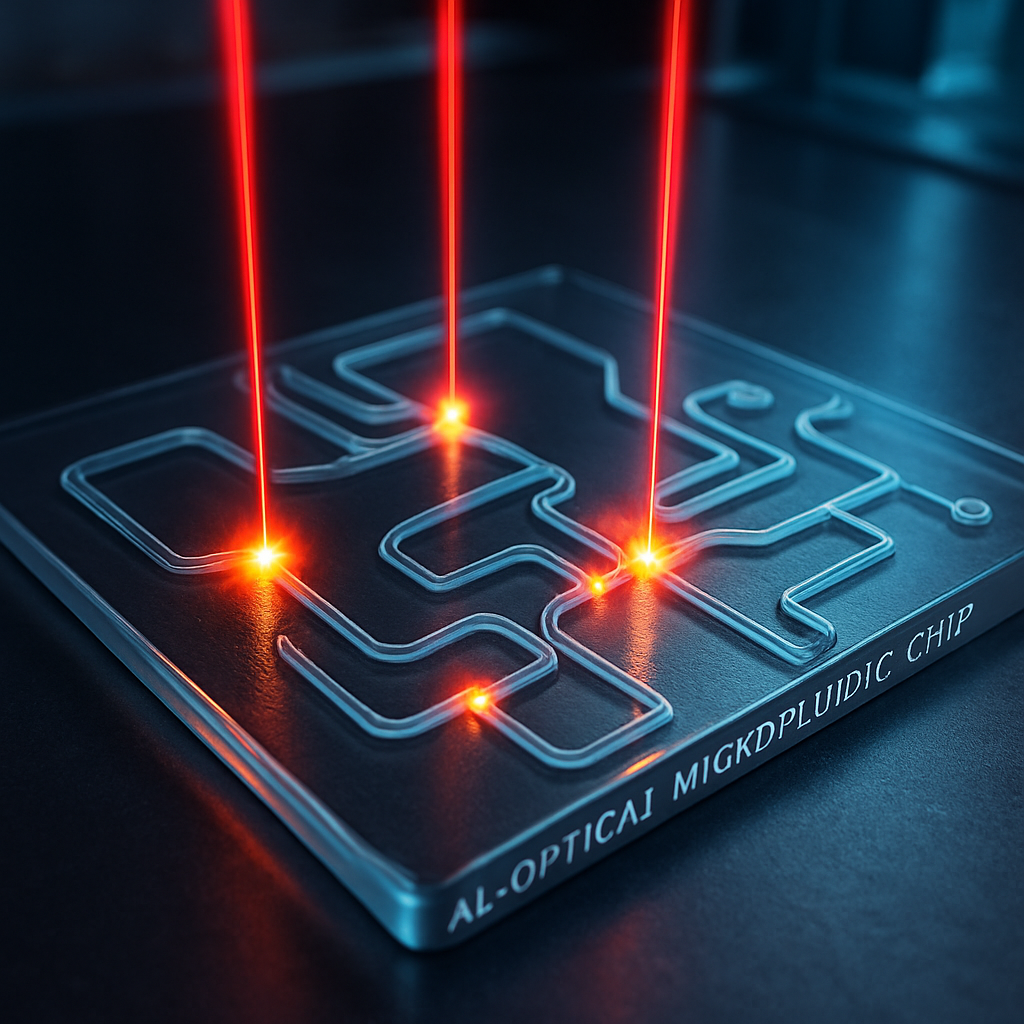
Conventional microfluidic systems, the "lab-on-a-chip" technologies that underpin much of modern biology and chemistry, rely on a hardware-based paradigm. Their channels, mixers, valves, and pumps are physically etched into materials like glass or PDMS, creating a fixed and immutable architecture. This approach, while powerful, suffers from inherent limitations: physical valves can fail, introduce dead volumes that trap precious samples, and the overall circuit is permanently locked to the function for which it was designed. A transformative new paradigm, all-optical microfluidics, fundamentally overcomes these constraints by replacing physical mechanisms with light. Using focused beams of light, it is now possible to create "virtual" or "photothermal" fluid control elements that are entirely non-invasive, instantly reconfigurable, and have no moving parts.
The core principle of this technology is the photothermal effect, where energy from a light source is absorbed by the fluid or the microchannel walls and converted into highly localized heat. This precise thermal manipulation induces powerful fluidic phenomena—from convection to phase changes—that can be harnessed to form on-demand pumps, valves, and mixers. This report explores the physics behind all-optical fluid control, its application in creating programmable fluidic circuits, and its revolutionary potential for high-throughput single-cell analysis, where the ability to gently and precisely manipulate individual cells is paramount.
The Physics of Photothermal Flow Control
The ability to push, pull, and block fluid using only light stems from the precise conversion of photons to thermal gradients. When a low-power laser is focused onto a microfluidic channel, the localized heating of the fluid generates flow through several distinct physical mechanisms. The most prominent is the Marangoni effect, where the temperature gradient creates a corresponding gradient in surface tension, inducing a strong convective flow that pulls fluid from the hot, low-tension region towards the cooler, high-tension surroundings. This effect is exceptionally potent for creating pumps and mixers within the channel. If the heating is sufficiently intense, a micro-bubble of vapor can be formed. The rapid expansion of this bubble acts as a powerful piston, displacing fluid, while its stable presence can serve as a perfect, zero-leakage temporary barrier, or a virtual valve.

The efficiency of this light-to-heat conversion is critical and is often enhanced by embedding plasmonic nanoparticles (e.g., gold nanorods) either directly into the fluid or onto the channel surfaces. These nanoparticles act as potent nano-heaters, concentrating optical energy and generating significant thermal effects with minimal input power. A crucial innovation in this area is the concept of the "Janus-Nanojet," a type of nanoparticle with asymmetric thermal properties. Such particles can be designed to absorb light and preferentially radiate heat in a specific direction, a feature that could be critical for manipulating biological cells by pushing them with a flow of warm buffer while shielding the cell itself from direct thermal stress (González-Colsa, J. et al., 2022).
Virtual Valving and Pumping: Towards Light-Programmable Fluidic Logic
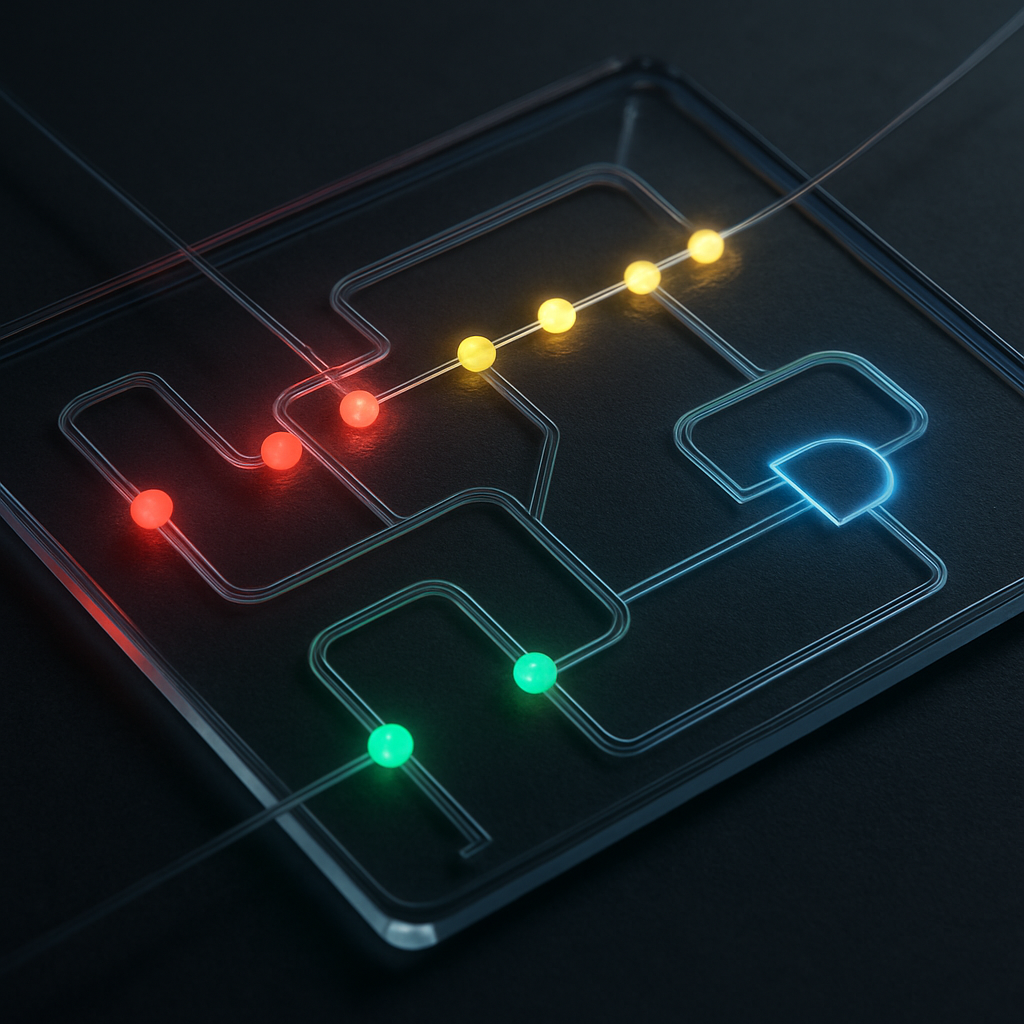
The true power of all-optical microfluidics lies not in creating a single valve or pump, but in orchestrating complex sequences of them in real-time. By using a digital projector or rapidly scanning lasers, it is possible to create arbitrary, dynamic patterns of light, effectively drawing a fluidic circuit into existence on a simple, channel-filled chip. A line of sequential laser spots activated in a peristaltic sequence becomes a powerful, pulseless pump. A focused spot at a channel junction becomes an instant, re-routable valve. This reconfigurability is analogous to the shift from fixed, hard-wired electrical circuits to programmable computer chips.
Building on this, we can hypothesize the creation of "photothermal fluidic logic gates." For example, a Y-shaped junction could function as an 'AND' gate, where a cell only proceeds down the central channel if two separate optical pumping signals are active in the input channels. By combining these virtual pumps (as signals) and valves (as gates), one could build complex, programmable microfluidic processors. This vision moves beyond static devices and towards the concept of reconfigurable micromachines (Zhang, S. et al., 2021), where the machine's function is defined not by its physical structure but by the controlling light field. A single, mass-produced microfluidic chip could be programmed by light to perform thousands of different experiments, dramatically lowering the cost and complexity of research.
Application Spotlight: Adaptive Single-Cell Sorting and Analysis
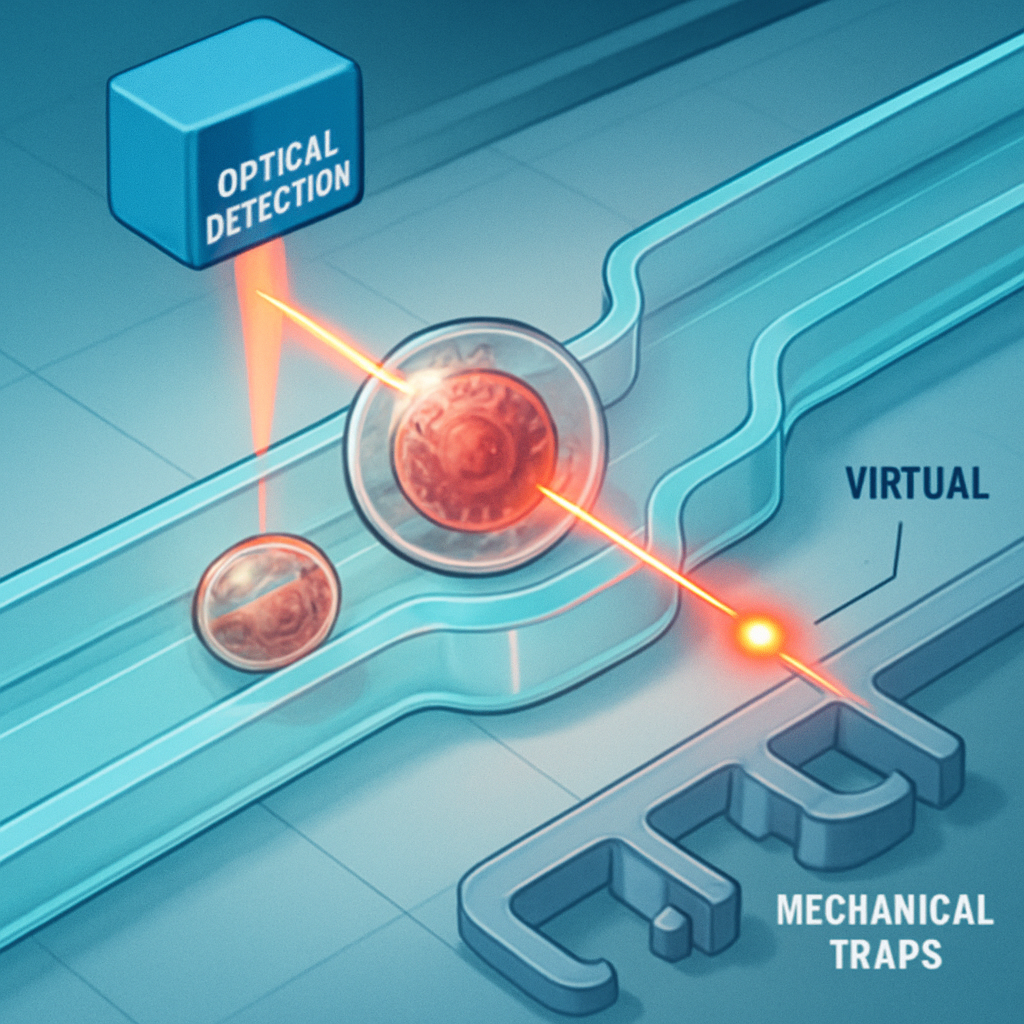
The gentle, non-contact nature of photothermal control makes it an ideal tool for single-cell analysis, a field where preserving cell viability is critical. Current methods like Fluorescence-Activated Cell Sorting (FACS) subject cells to high pressures and shear stresses. An all-optical sorter, in contrast, can analyze and divert cells with unmatched gentleness. As a cell flows down a channel, a detection laser identifies a target (e.g., a cancerous cell tagged with a fluorescent marker). A fraction of a second later, a downstream control laser activates a virtual valve, creating a temporary thermal barrier that gently nudges the target cell into a collection channel, a process far more delicate than mechanical sorting (Yang, M. et al., 2024).
Beyond sorting, optical control enables trapping and manipulation. A ring of light can create a thermal trap, holding a single cell stationary for extended microscopy without any physical contact, allowing researchers to observe its behavior over time. We can further propose the concept of adaptive experimentation. An AI monitoring the cell's response (e.g., via a biosensor for metabolic activity) could reprogram the light field in real-time. If a cell shows resistance to one drug delivered by a virtual pump, the AI could instantly reroute the flow, flush the channel, and apply a different compound. This creates a closed feedback loop between analysis and manipulation, a feat impossible with fixed-channel devices and a core principle for the future of autonomous robotic science (Medany, M. et al., 2025).
Conclusion
All-optical microfluidics is poised to transition from a laboratory curiosity to a foundational technology for biology and medicine. The primary challenge remains thermal management: creating strong enough flows without compromising the viability of sensitive biological samples like cardiomyocytes or stem cells (Wang, W. et al., 2024). This will be addressed through the development of advanced photothermal materials, optimized laser pulsing strategies, and clever device designs that isolate cells from the hottest zones.
Looking forward, the true frontier is the integration of these reconfigurable optical systems with artificial intelligence to create what could be termed "4D Microfluidics." The fourth dimension is not just time, but dynamic function. A simple, inexpensive chip of microchannels becomes a blank slate; the light field is the software that defines its purpose. An AI could learn the optimal optical patterns to sort rare cells, guide neurons to form connections, or perform multi-step chemical syntheses, reprogramming the device's function on the fly. This represents a fundamental shift from designing a chip for a task to designing a task for a chip, heralding an era of intelligent, non-invasive, and endlessly adaptable "virtual laboratories."
References
- González-Colsa, J., Franco, A., Bresme, F., Moreno, F., & Albella, P. (2022). Janus-Nanojet as an efficient asymmetric photothermal source. Scientific Reports. https://doi.org/10.1038/s41598-022-17630-0
- Medany, M., Piglia, L., Achenbach, L., Mukkavilli, S. K., & Ahmed, D. (2025). Model-based reinforcement learning for ultrasound-driven autonomous microrobots. Nature Machine Intelligence. https://doi.org/10.1038/s42256-025-01054-2
- Wang, W., Su, W., Han, J., Song, W., Li, X., Xu, C., Sun, Y., & Wang, L. (2024). Microfluidic platforms for monitoring cardiomyocyte electromechanical activity. Microsystems & Nanoengineering. https://doi.org/10.1038/s41378-024-00751-z
- Wu, K.-H., Zhu, L.-T., Xiao, F.-F., Hu, X., Li, S.-S., & Chen, L.-J. (2024). Light-regulated soliton dynamics in liquid crystals. Nature Communications. https://doi.org/10.1038/s41467-024-51383-w
- Yang, M., Shi, Y., Song, Q., Wei, Z., Dun, X., Wang, Z., Wang, Z., Qiu, C.-W., Zhang, H., & Cheng, X. (2024). Optical sorting: past, present and future. Light: Science & Applications. https://doi.org/10.1038/s41377-024-01734-5
- Zhang, S., Elsayed, M., Peng, R., Chen, Y., Zhang, Y., Peng, J., Li, W., Chamberlain, M. D., Nikitina, A., Yu, S., Liu, X., Neale, S. L., & Wheeler, A. R. (2021). Reconfigurable multi-component micromachines driven by optoelectronic tweezers. Nature Communications. https://doi.org/10.1038/s41467-021-25582-8